The theragnostic advances of exosomes in managing leukaemia
- PMID: 39659020
- PMCID: PMC11632122
- DOI: 10.1111/jcmm.70052
The theragnostic advances of exosomes in managing leukaemia
Abstract
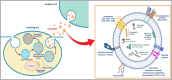
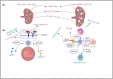
Leukaemia, a group of haematological malignancies, presents ongoing diagnosis, prognosis, and treatment challenges. A major obstacle in treating this disease is the development of drug resistance. Overcoming drug resistance poses a significant barrier to effective leukaemia treatment. The emergence of exosome research has unveiled new insights into the probable theragnostic implementations in leukaemia. Various research has exhibited the diagnostic possibilities of exosomes in identifying leukaemia-specific biomarkers, including genetic mutations and fusion transcripts. Additionally, exosomes have been implicated in disease progression and treatment response, rendering them appealing targets for therapeutics. Exosomes, originating from diverse cell types, are instrumental in intercellular communication as they participate in the functional transportation of molecules like proteins, nucleic acids and lipids across space. Exosomes have a dual role in immune regulation, mediating immune suppression and modulating anti-leukaemia immune responses. Interestingly, exosomes can even act as drug transport vehicles. This review delves into the intricate process of exosome biogenesis, shedding light on their formation and release from donor cells. The key mechanisms engaged in exosome biogenesis, for instance, the endosomal sorting complexes required for transport (ESCRT) machinery and ESCRT-independent pathways, are thoroughly discussed. Looking ahead, future approaches that leverage innovative technologies hold the promise of revolutionizing disease management and improving patient outcomes.
Keywords: biogenesis; drug resistance; exosomes; leukaemia; therapeutics.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1). doi: 10.1080/20013078.2018.1535750 - DOI - PMC - PubMed
-
- Renaud S, Lefebvre A, Moralès O, Delhem N. Mini review: exosomes from discovery to isolation. Biomed J Sci Tech Res. 2019;15(2):11286‐11293. doi: 10.26717/BJSTR.2019.15.002683 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

