Gut microbiota-mediated gut-liver axis: a breakthrough point for understanding and treating liver cancer
- PMID: 39659059
- PMCID: PMC12016628
- DOI: 10.3350/cmh.2024.0857
Gut microbiota-mediated gut-liver axis: a breakthrough point for understanding and treating liver cancer
Abstract
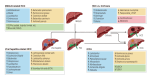
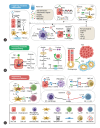
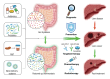
The trillions of commensal microorganisms living in the gut lumen profoundly influence the physiology and pathophysiology of the liver through a unique gut-liver axis. Disruptions in the gut microbial communities, arising from environmental and genetic factors, can lead to altered microbial metabolism, impaired intestinal barrier and translocation of microbial components to the liver. These alterations collaboratively contribute to the pathogenesis of liver disease, and their continuous impact throughout the disease course plays a critical role in hepatocarcinogenesis. Persistent inflammatory responses, metabolic rearrangements and suppressed immunosurveillance induced by microbial products underlie the pro-carcinogenic mechanisms of gut microbiota. Meanwhile, intrahepatic microbiota derived from the gut also emerges as a novel player in the development and progression of liver cancer. In this review, we first discuss the causes of gut dysbiosis in liver disease, and then specify the pivotal role of gut microbiota in the malignant progression from chronic liver diseases to hepatobiliary cancers. We also delve into the cellular and molecular interactions between microbes and liver cancer microenvironment, aiming to decipher the underlying mechanism for the malignant transition processes. At last, we summarize the current progress in the clinical implications of gut microbiota for liver cancer, shedding light on microbiota-based strategies for liver cancer prevention, diagnosis and therapy.
Keywords: Clinical translation; Gut microbiota; Gut-liver axis; Intratumoral microbiota; Liver cancer.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

References
-
- Pabst O, Hornef MW, Schaap FG, Cerovic V, Clavel T, Bruns T. Gut-liver axis: barriers and functional circuits. Nat Rev Gastroenterol Hepatol. 2023;20:447–461. - PubMed
-
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. - PubMed
-
- Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

