Duration of sodium zirconium cyclosilicate treatment and continuation of RAASi therapy after a hyperkalaemia episode
- PMID: 39659133
- PMCID: PMC12055344
- DOI: 10.1002/ehf2.15171
Duration of sodium zirconium cyclosilicate treatment and continuation of RAASi therapy after a hyperkalaemia episode
Abstract
Aims: Renin-angiotensin-aldosterone system inhibitors (RAASi) are foundational in the management of heart failure (HF) and chronic kidney disease (CKD) but increase the risk of hyperkalaemia. To facilitate continuation of RAASi therapy, guidelines suggest managing hyperkalaemia using newer potassium binders such as sodium zirconium cyclosilicate (SZC). This observational study describes the likelihood of continued RAASi therapy by duration of SZC treatment.
Methods: The study population included non-dialysis-dependent adults diagnosed with HF and/or CKD who initiated outpatient SZC treatment while receiving RAASi therapy. Patients were identified using healthcare registers and claims data from the United States, Japan and Spain. SZC treatment duration was described using the Kaplan-Meier method. Hernán's clone-censor-weight (CCW) approach, using principles of trial emulation, was applied to evaluate the likelihood of continued RAASi therapy at specific time points by distinct SZC treatment durations, using a weighted Kaplan-Meier method and Z-tests.
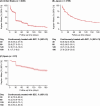
Results: The study included 7980 patients, from the United States (n = 4849), Japan (n = 2759) and Spain (n = 372). Across the three countries, mean patient age was 73.1-75.0 years, 53.2%-66.4% of patients were male, 39.0%-75.0% had HF and 76.9%-95.3% had CKD. Between Days 30 and 120, the percentage of patients remaining on SZC treatment decreased from 36.5% to 12.8% in the United States, from 63.8% to 33.7% in Japan, and from 81.9% to 65.0% in Spain. In the United States, patients who continued SZC treatment beyond 30 days had a higher likelihood of continuing RAASi therapy for up to 90 days (P < 0.001), and continuing SZC treatment beyond 60 days was superior for continuing RAASi therapy for up to 6 months (P < 0.001), versus earlier SZC discontinuation. At 120 days, the likelihood of remaining on RAASi therapy was 69%-70% for SZC treatment durations exceeding 60 days, versus 59% for shorter durations (1-30 days) (P < 0.001). Similar patterns were observed in Japan. At 120 days, the likelihood of remaining on RAASi therapy was 86%-87% for SZC treatment durations exceeding 90 days, versus 82% for shorter SZC treatment durations (1-30 days) (P < 0.05). The CCW analyses were not deemed feasible in the Spanish dataset due to the smaller initial sample size and few patients having a relatively short SZC treatment duration.
Conclusions: Patients with longer SZC treatment experience sustained protection against RAASi discontinuation, and the risk of RAASi discontinuation resumes once SZC is discontinued.
Keywords: chronic kidney disease; heart failure; hyperkalaemia; potassium binder; renin–angiotensin–aldosterone system inhibitor; sodium zirconium cyclosilicate.
© 2024 The Author(s). ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
C. V. P. has received consultancy fees from AstraZeneca. D. A. has received lecture fees and/or consultation fees from AstraZeneca, Bayer, Boehringer Ingelheim, CSL Vifor, Eli Lilly, GSK, Menarini, Otsuka and Viatris. E. K. reports no disclosures. I. J. S. L. is an employee of Hospital Universitari i Politècnic La Fe and has served on the speakers' bureaus of AstraZeneca and Novartis. E. L., S. F., C. M. G. and A. L. are employees and stockholders of AstraZeneca. T. M. has received honoraria for lectures from AstraZeneca. A. R. has received research grants and consulting fees from AstraZeneca.
Figures


References
-
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4‐131. doi: 10.1002/ejhf.2333 - DOI - PubMed
-
- Hsu TW, Liu JS, Hung SC, Kuo KL, Chang YK, Chen YC, et al. Renoprotective effect of renin–angiotensin–aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med 2014;174:347‐354. doi: 10.1001/jamainternmed.2013.12700 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
