A Bayesian hierarchical model for disease mapping that accounts for scaling and heavy-tailed latent effects
- PMID: 39659172
- PMCID: PMC11874469
- DOI: 10.1177/09622802241293776
A Bayesian hierarchical model for disease mapping that accounts for scaling and heavy-tailed latent effects
Abstract
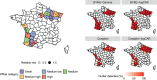
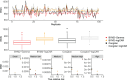
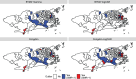
In disease mapping, the relative risk of a disease is commonly estimated across different areas within a region of interest. The number of cases in an area is often assumed to follow a Poisson distribution whose mean is decomposed as the product between an offset and the logarithm of the disease's relative risk. The log risk may be written as the sum of fixed effects and latent random effects. A modified Besag-York-Mollié (BYM2) model decomposes each latent effect into a weighted sum of independent and spatial effects. We build on the BYM2 model to allow for heavy-tailed latent effects and accommodate potentially outlying risks, after accounting for the fixed effects. We assume a scale mixture structure wherein the variance of the latent process changes across areas and allows for outlier identification. We propose two prior specifications for this scale mixture parameter. These are compared through various simulation studies and in the analysis of Zika cases from the first (2015-2016) epidemic in Rio de Janeiro city, Brazil. The simulation studies show that the proposed model always performs at least as well as an alternative available in the literature, and often better, both in terms of widely applicable information criterion, mean squared error and of outlier identification. In particular, the proposed parametrisations are more efficient, in terms of outlier detection, when outliers are neighbours. Our analysis of Zika cases finds 23 out of 160 districts of Rio as potential outliers, after accounting for the socio-development index. Our proposed model may help prioritise interventions and identify potential issues in the recording of cases.
Keywords: BYM2 model; Zika virus infection; outliers; scale mixture; spatial statistics; vector-borne disease.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- World Health Organization. Vector-borne diseases. 2020, https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases.
-
- Nogueira RMR, Miagostovich MP, Schatzmayr HG, et al. Dengue in the state of Rio de Janeiro, Brazil, 1986–1998. Memórias do Instituto Oswaldo Cruz 1999; 94: 297–304. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

