Screening and diagnosis of pulmonary hypertension associated with chronic lung disease (PH-CLD): A consensus statement from the pulmonary vascular research institute's innovative drug development initiative-group 3 pulmonary hypertension
- PMID: 39659477
- PMCID: PMC11629413
- DOI: 10.1002/pul2.70005
Screening and diagnosis of pulmonary hypertension associated with chronic lung disease (PH-CLD): A consensus statement from the pulmonary vascular research institute's innovative drug development initiative-group 3 pulmonary hypertension
Abstract
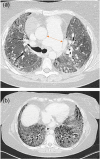
Pulmonary hypertension (PH) is a frequent complication of chronic lung disease (CLD). However, PH is difficult to diagnose early since accompanying symptoms overlap and are similar to those of the underlying CLD. In most cases the PH is mild to moderate and therefore physical signs may be absent or subtle. This consensus paper provides insight into the clues that might suggest the presence of occult PH in patients with CLD. An overview of current diagnostic tools and emerging diagnostic technologies is provided as well as guidance for the work-up and diagnosis of PH in patients with CLD.
Keywords: chronic lung disease; diagnosis; imaging; pulmonary hypertension; screening.
© 2024 The Author(s). Pulmonary Circulation published by John Wiley & Sons Ltd on behalf of Pulmonary Vascular Research Institute.
Conflict of interest statement
Dr. Vitulo has consulted for MSD and AOP and has received support for attending congresses from MSD, Janssen all of which not related to this manuscript. Dr. Piccari has received research funding from and served as a speaker for Janssen and Ferrer, advised Janssen, Ferrer and United Therapeutics as well as received support for attending congresses from Janssen, MSD and Ferrer, all of which not related to this manuscript. Dr. S. John Wort received honoraria from Janssen, MSD, Bayer, Ferrer and Acceleron for advisory boards, received honoraria from Janssen for educational activity, received unrestricted research grants from Janssen, Ferrer and Bayer, and travel grants, conference registration and accommodation from Janssen and Ferrer. Dr. Shlobin has consulted for UT, Bayer, ALtavant, Aerovate, Jenssen&Jenssen Jenssen& Jenssen and Merck, and is on the speaker bureau for UT, Bayer, and J&J; Dr. Stockbridge has no conflict of interest. Dr. Kovacs reports personal fees and nonfinancial support from Actelion, Janssen, Bayer, GSK, MSD, Boehringer Ingelheim, Novartis, Chiesi, Vitalaire, Ferrer, and AOP outside the submitted work. Dr. Vizza has no conflict of interest regarding this topic. Dr. Hassoun serves on a scientific advisory board for Merck, an activity unrelated to the current work. Dr. Olschewski received funding from Actelion, AOP, Astra Zeneca, Bayer, Boehringer, Chiesi, GSK, Iqvia, Janssen, Menarini, MSD, Novartis, Ludwig Boltzmann Society, Ferrer, MedUpdate, and Mondial not related to this work. Dr. Girgis received honoraria from Boehringer Ingelheim and research funding from UT. Dr. Nikkho is an employee of Bayer AG.Dr. Nathan is a consultant for United Therapeutics, Bellerophon, Third Pole, Roche, Boehringer‐Ingelheim, Insmed, Roivant, Merck and Daewoong. He is on the speakers bureau for United Therapeutics and Boehringer‐Ingelheim. He is also on the Gossamer Bio Board of Directors.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

