Association between the systemic immune-inflammation index and the outcome of liver fibrosis in patients with chronic hepatitis C
- PMID: 39659620
- PMCID: PMC11628305
- DOI: 10.3389/fmed.2024.1486503
Association between the systemic immune-inflammation index and the outcome of liver fibrosis in patients with chronic hepatitis C
Abstract
Background: Risk factors that influence the outcome of patients with chronic hepatitis C (CHC) are not fully understood. The systemic immune-inflammatory index (SII) is an independent prognostic factor for multiple diseases. However, the impact of the SII on the outcome of liver fibrosis is unclear.
Methods: This prospective real-world study enrolled patients with CHC treated with sofosbuvir/velpatasvir. Logistic regression models were used to investigate the relationship between the SII and the outcome of liver fibrosis in treatment-naive patients. Liver fibrosis was assessed using aspartate aminotransferase-to-platelet ratio index (APRI) and fibrosis-4 index (FIB-4).
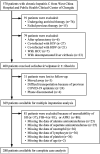
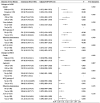
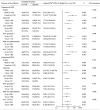
Results: Of the 288 participants, the SII was 238.2 (153.0-358.2). The non-improved outcomes of liver fibrosis assessed with APRI (non-improved APRI) and FIB-4 (non-improved FIB-4) were 83.0 and 87.5%, respectively. Adjusted models showed that the SII was positively associated with non-improved APRI (adjusted OR (95% CI): 1.013 (1.009-1.017), p < 0.001) and FIB-4 (adjusted OR (95% CI): 1.004 (1.001-1.007), p = 0.012). Similarly, a higher SII was associated with a higher risk of non-improved APRI (adjusted OR (95% CI): 13.53 (5.60-32.68), p < 0.001) and FIB-4 (adjusted OR (95% CI): 5.69 (2.17-14.90), p < 0.001). The association with non-improved APRI was much more remarkable in patients with alanine aminotransferase <2 ULN, and the association with non-improved FIB-4 was remarkable in patients aged <50 years. Multiple imputation analyses confirmed the robustness of these results.
Conclusion: Our findings suggested that the SII was positively associated with non-improved outcomes of liver fibrosis in patients with CHC. These results need to be validated in large-scale prospective cohorts.
Keywords: chronic hepatitis C; liver fibrosis; outcome; risk factor; systemic immune-inflammation index.
Copyright © 2024 Ma, Wang, Du and Tang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures


Similar articles
-
Fibrosis index based on four factors better predicts advanced fibrosis or cirrhosis than aspartate aminotransferase/platelet ratio index in chronic hepatitis C patients.J Formos Med Assoc. 2015 Oct;114(10):923-8. doi: 10.1016/j.jfma.2015.07.004. Epub 2015 Aug 13. J Formos Med Assoc. 2015. PMID: 26279173
-
The applicability of non-invasive methods for assessing liver fibrosis in hemodialysis patients with chronic hepatitis C.PLoS One. 2020 Nov 20;15(11):e0242601. doi: 10.1371/journal.pone.0242601. eCollection 2020. PLoS One. 2020. PMID: 33216807 Free PMC article.
-
Diagnostic value of FIB-4, aspartate aminotransferase-to-platelet ratio index and liver stiffness measurement in hepatitis B virus-infected patients with persistently normal alanine aminotransferase.World J Gastroenterol. 2017 Aug 21;23(31):5746-5754. doi: 10.3748/wjg.v23.i31.5746. World J Gastroenterol. 2017. PMID: 28883700 Free PMC article.
-
Gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid-enhanced magnetic resonance imaging for evaluating fibrosis regression in chronic hepatitis C patients after direct-acting antiviral.World J Gastroenterol. 2022 May 28;28(20):2214-2226. doi: 10.3748/wjg.v28.i20.2214. World J Gastroenterol. 2022. PMID: 35721884 Free PMC article.
-
Diagnostic value of combined serum biomarkers for the evaluation of liver fibrosis in chronic hepatitis C infection: A multicenter, noninterventional, observational study.Turk J Gastroenterol. 2018 Jul;29(4):464-472. doi: 10.5152/tjg.2018.16597. Turk J Gastroenterol. 2018. PMID: 30249562 Free PMC article.
Cited by
-
Mediating role of systemic immune-inflammation index between heavy metal exposure and hepatic steatosis/hepatic fibrosis: evidence from NHANES 2005-2020.Front Nutr. 2025 May 21;12:1566345. doi: 10.3389/fnut.2025.1566345. eCollection 2025. Front Nutr. 2025. PMID: 40469666 Free PMC article.
References
LinkOut - more resources
Full Text Sources

