Synaptic Structure and Transcriptomic Profiling of Reward and Sensory Brain Areas in Male Mice of Fentanyl Addiction
- PMID: 39659661
- PMCID: PMC11630728
- DOI: 10.2147/SAR.S484167
Synaptic Structure and Transcriptomic Profiling of Reward and Sensory Brain Areas in Male Mice of Fentanyl Addiction
Abstract
Background: Opioid-based medications are powerful analgesics commonly prescribed for pain management, but they are also highly addictive. The over-prescription of opioids analgesics has triggered current opioid crisis, which now has expanded to heroin and illicit synthetic opioids like fentanyl and its analogues. The side effects of fentanyl abuse have been well recognized, yet the underlying molecular adaptations across brain regions upon fentanyl exposure remain elusive.
Methods: The transmission electron microscopy (TEM) and next-generation RNA-sequencing (RNA-seq) were used to investigate the ultrastructure synaptic alterations and transcriptional profiling changes of reward and sensory brain regions in mice after fentanyl exposure.
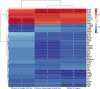
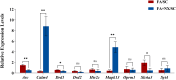
Results: The naloxone-precipitated acute withdrawal symptoms were observed in mice exposed to fentanyl. Results of TEM showed an increase in the number of synapses, widening of synaptic gaps, and thickening of postsynaptic density in the NAc of the fentanyl addiction mice, accompanied by obvious mitochondrial swelling. RNA-seq identified differentially expressed genes (DEGs) in prefrontal cortex of mice brains after fentanyl exposure, and the expression of some addiction-related genes such as Calm4, Cdh1, Drd1/2/3/4, F2rl2, Gabra6, Ht2cr, Oprk1 and Rxfp3 showed the most striking changes among experimental groups. KEGG enrichment analysis indicated that these DEGs were related to the development of addiction behavior, dopaminergic/GABAergic/serotonergic synapse, synapse assembly/synaptic plasticity/synaptic vesicle cycle, cAMP/MAPK signaling pathway, neuroactive ligand-receptor interactions. These transcriptomic changes may be correlated with the structural and behavioral changes observed in fentanyl-exposed mice.
Discussion: The findings of this study contribute to a better understanding of the molecular mechanism of addiction behavior, which is essential for the development of optimized therapy strategies for addicts.
Keywords: addiction; fentanyl; gene expression; synaptic plasticity; transcriptional profiles.
© 2024 Feng et al.
Conflict of interest statement
The authors declare no conflict of interest in this work.
Figures


Similar articles
-
Transcriptomic profiling of reward and sensory brain areas in perinatal fentanyl exposed juvenile mice.Neuropsychopharmacology. 2023 Nov;48(12):1724-1734. doi: 10.1038/s41386-023-01639-8. Epub 2023 Jul 3. Neuropsychopharmacology. 2023. PMID: 37400565 Free PMC article.
-
Differential Patterns of Synaptic Plasticity in the Nucleus Accumbens Caused by Continuous and Interrupted Morphine Exposure.J Neurosci. 2023 Jan 11;43(2):308-318. doi: 10.1523/JNEUROSCI.0595-22.2022. Epub 2022 Nov 17. J Neurosci. 2023. PMID: 36396404 Free PMC article.
-
Perinatal Fentanyl Exposure Leads to Long-Lasting Impairments in Somatosensory Circuit Function and Behavior.J Neurosci. 2021 Apr 14;41(15):3400-3417. doi: 10.1523/JNEUROSCI.2470-20.2020. J Neurosci. 2021. PMID: 33853934 Free PMC article.
-
Glutamatergic synaptic plasticity in the mesocorticolimbic system in addiction.Front Cell Neurosci. 2015 Jan 20;8:466. doi: 10.3389/fncel.2014.00466. eCollection 2014. Front Cell Neurosci. 2015. PMID: 25653591 Free PMC article. Review.
-
Estimating naloxone need in the USA across fentanyl, heroin, and prescription opioid epidemics: a modelling study.Lancet Public Health. 2022 Mar;7(3):e210-e218. doi: 10.1016/S2468-2667(21)00304-2. Epub 2022 Feb 10. Lancet Public Health. 2022. PMID: 35151372 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

