Microneedle technology for enhanced topical treatment of skin infections
- PMID: 39659727
- PMCID: PMC11629152
- DOI: 10.1016/j.bioactmat.2024.11.027
Microneedle technology for enhanced topical treatment of skin infections
Abstract
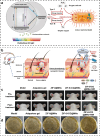
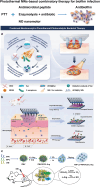
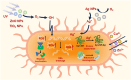
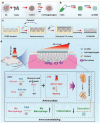
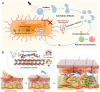
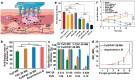
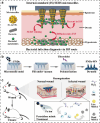
Skin infections caused by microbes such as bacteria, fungi, and viruses often lead to aberrant skin functions and appearance, eventually evolving into a significant risk to human health. Among different drug administration paradigms for skin infections, microneedles (MNs) have demonstrated superiority mainly because of their merits in enhancing drug delivery efficiency and reducing microbial resistance. Also, integrating biosensing functionality to MNs offers point-of-care wearable medical devices for analyzing specific pathogens, disease status, and drug pharmacokinetics, thus providing personalized therapy for skin infections. Herein, we do a timely update on the development of MN technology in skin infection management, with a special focus on how to devise MNs for personalized antimicrobial therapy. Notably, the advantages of state-of-the-art MNs for treating skin infections are pointed out, which include hijacking sequential drug transport barriers to enhance drug delivery efficiency and delivering various therapeutics (e.g., antibiotics, antimicrobial peptides, photosensitizers, metals, sonosensitizers, nanoenzyme, living bacteria, poly ionic liquid, and nanomoter). In addition, the nanoenzyme-based multimodal antimicrobial therapy is highlighted in addressing intractable infectious wounds. Furthermore, the MN-based biosensors used to identify pathogen types, track disease status, and quantify antibiotic concentrations are summarized. The limitations of antimicrobial MNs toward clinical translation are offered regarding large-scale production, quality control, and policy guidance. Finally, the future development of biosensing MNs with easy-to-use and intelligent properties and MN-based wearable drug delivery for home-based therapy are prospected. We hope this review will provide valuable guidance for future development in MN-mediated topical treatment of skin infections.
Keywords: Biosensing; Drug delivery systems; Microneedle; Skin infection; Topical treatment.
© 2024 The Authors.
Conflict of interest statement
All authors declare that there is no conflict of interest in this paper.
Figures






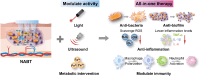



References
-
- Chin J.S., Madden L., Chew S.Y., Becker D.L. Drug therapies and delivery mechanisms to treat perturbed skin wound healing. Adv. Drug Deliv. Rev. 2019;149–150:2–18. - PubMed
-
- Anjani Q.K., Sabri A.H.B., Hutton A.J., Cárcamo-Martínez Á., Wardoyo L.A.H., Mansoor A.Z., Donnelly R.F. Microarray patches for managing infections at a global scale. J. Contr. Release. 2023;359:97–115. - PubMed
-
- Phatale V., Vaiphei K.K., Jha S., Patil D., Agrawal M., Alexander A. Overcoming skin barriers through advanced transdermal drug delivery approaches. J. Contr. Release. 2022;351:361–380. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

