Refractory phenotype of Aspergillus-sensitized asthma with bronchiectasis and allergic bronchopulmonary aspergillosis
- PMID: 39659740
- PMCID: PMC11629325
- DOI: 10.1016/j.jacig.2024.100364
Refractory phenotype of Aspergillus-sensitized asthma with bronchiectasis and allergic bronchopulmonary aspergillosis
Abstract
Background: Sensitization to Aspergillus, mucus plugs, and bacterial colonization may coexist and relate to a refractory phenotype during follow-up in asthma with bronchiectasis and allergic bronchopulmonary aspergillosis (ABPA).
Objective: This study aimed to clarify the features of Aspergillus-sensitized refractory asthma with bronchiectasis and determine the refractory phenotype in this population and ABPA.
Methods: This study included cases of the oldest available Aspergillus fumigatus-specific IgE data and chest computed tomography images from a nationwide survey of refractory asthma with bronchiectasis. The characteristics of the A fumigatus-IgE positive (Af sIgE+) group were investigated and compared with its nonsensitized counterpart (Af sIgE-) and ABPA group. Cluster analysis was conducted to determine the refractory phenotype.
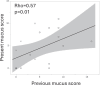
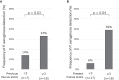
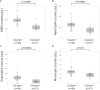
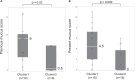
Results: The Af sIgE+ group (n = 35) demonstrated type 2 inflammation levels intermediate between the ABPA (n = 42) and Af sIgE- (n = 38) groups while exhibiting higher blood monocyte counts than the Af sIgE- group. Cluster analysis conducted in patients with ABPA and Af sIgE+ newly determined 2 clusters: one was characterized by a younger age of asthma onset with fungal detection in sputum, and the other was characterized by mucus plugs and inflammation with eosinophils and monocytes, which was significantly related to mucus plugs, airflow limitation, and trend to show exacerbation. In the latter cluster, mucus plugs persisted, and 30% yielded Pseudomonas aeruginosa in the sputum <5 years later.
Conclusion: The refractory phenotype with persistent mucus plugs was identified in Aspergillus-sensitized refractory asthma with bronchiectasis and ABPA. Mucus plug prevention is warranted.
Keywords: ABPA; Aspergillus sensitization; bronchiectasis; monocyte; mucus plug; refractory asthma.
© 2024 The Author(s).
Conflict of interest statement
Supported by the Scientific Assembly of Allergy, Immunology & Inflammation, 10.13039/501100009098Japanese Respiratory Society, 10.13039/100030831Novartis Japan, and the 10.13039/100009619Japan Agency for Medical Research and Development (research grant 24ek0410097 for Allergic Disease and Immunology). Disclosure of potential conflict of interest: K. Asano received lecturer fees from Sanofi, AstraZeneca, and Boehringer Ingelheim outside this work; and received a research grant on Allergic Disease and Immunology from the Japan Agency for Medical Research and Development. K. Fukunaga received lecturer fees from Sanofi, AstraZeneca, GlaxoSmithKline, Kyorin Pharmaceutical, Boehringer Ingelheim, and Novartis Pharma outside this work; and received grants from Boehringer Ingelheim and Chugai Pharmaceutical outside this work. N. Harada received lecturer fees from Sanofi, AstraZeneca, GlaxoSmithKline, Kyorin Pharmaceutical, and Novartis Pharma outside this work; and royalties from Sanofi, AstraZeneca, Daikin Investment, and TOSOH. T. Hirai received lecturer fees from AstraZeneca, Kyorin Pharmaceutical, and Boehringer Ingelheim outside this work. N. Hattori received lecturer fees from Sanofi, AstraZeneca, GlaxoSmithKline, Kyorin Pharmaceutical, Ono Pharmaceutical, MSD, and Pfizer Japan outside this work. T. Kimura received lecture fees from Sanofi, AstraZeneca, GlaxoSmithKline, Eli Lilly Japan, Chugai Phamaceutical, Novartis Pharma, Brsitol Myers Squibb, Meiji Seika Pharma, DAIICHI SANKYO, and MSD outside this work. H. Matsumoto received lecturer fees from Sanofi, AstraZeneca, GlaxoSmithKline, Kyorin Pharmaceutical, and Boehringer Ingelheim; received grants from Kyorin Pharmaceutical, Boehringer Ingelheim, and Teijin Pharma outside this work; and received support from the Japanese Respiratory Society and a research grant from Novartis Japan. O. Matsuno received lecturer fees from Sanofi, AstraZeneca, and GlaxoSmithKline. T. Sakagami received lecturer fees from AstraZeneca, GlaxoSmithKline, Novartis Pharma, and Boehringer Ingelheim outside this work. H. Sugiura received lecturer fees from Sanofi, AstraZeneca, GlaxoSmithKline, Novartis Pharma, and Boehringer Ingelheim outside this work. H. Sunadome reports grants from Philips Japan, ResMed, Fukuda Denshi, and Fukuda Lifetec Keiji outside this work. N. Tanabe received research grants from Daiichi Sankyo and FUJIFILM outside this work. K. Tomii received lecturer fees from Sanofi, AstraZeneca, GlaxoSmithKline, and Novartis Pharma outside this work. A. Yokoyama received lecturer fees from Sanofi, AstraZeneca, GlaxoSmithKline, and Boehringer Ingelheim outside this work. The rest of the authors declare that they have no relevant conflicts of interest.
Figures


References
-
- Masaki K., Fukunaga K., Matsusaka M., Kabata H., Tanosaki T., Mochimaru T., et al. Characteristics of severe asthma with fungal sensitization. Ann Allergy Asthma Immunol. 2017;119:253–257. - PubMed
-
- Bendien S.A., van Loon-Kooij S., Kramer G., Huijgen W., Altenburg J., Ten Brinke A., et al. 2020. Bronchiectasis in severe asthma: does it make a difference? Respiration; pp. 1–9. - PubMed
-
- Mistry H., Ajsivinac Soberanis H.M., Kyyaly M.A., Azim A., Barber C., Knight D., et al. The clinical implications of Aspergillus fumigatus sensitization in difficult-to-treat asthma patients. J Allergy Clin Immunol Pract. 2021;9:4254–4267.e10. - PubMed
-
- Sehgal I.S., Dhooria S., Prasad K.T., Muthu V., Aggarwal A.N., Rawat A., et al. Sensitization to A fumigatus in subjects with non–cystic fibrosis bronchiectasis. Mycoses. 2021;64:412–419. - PubMed
LinkOut - more resources
Full Text Sources

