Recommendations for the effective use of T-cell-redirecting therapies: a Canadian consensus statement
- PMID: 39659785
- PMCID: PMC11628543
- DOI: 10.3389/fonc.2024.1446995
Recommendations for the effective use of T-cell-redirecting therapies: a Canadian consensus statement
Abstract
Background: T-cell-redirecting therapies, such as bispecific antibodies and chimeric antigen receptor T-cells, exploit the cytotoxic capabilities of the immune system to destroy cells expressing specific surface antigens, including malignant cells. These therapies have demonstrated unprecedented rates, depth, and duration of responses in relapsed and refractory multiple myeloma. However, there are significant challenges in implementing these therapies into practice, which require multidisciplinary and multicenter coordination and significant healthcare resources to effectively manage these patients. So far, there are no Canadian guidelines for the effective implementation and use of T-cell-redirecting therapies.
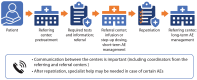
Methods: This consensus statement was developed based on three advisory meetings held in March, July, and November 2023. During these meetings, a panel of Canadian subject matter experts and representation from Myeloma Canada gathered to discuss the optimal procedures for the use of T-cell-redirecting therapies in the treatment of multiple myeloma. Members of the panel performed a thorough review of randomized clinical trials, real-world data, and other current literature, and provided their up-to-date clinical experience with T-cell-redirecting therapies in Canadian practice settings. Subsequently, asynchronous working groups were appointed to develop unified criteria for patient selection, appraise referral pathways, and devise strategies for management of short-term and long-term adverse events arising from the use of T-cell-redirecting therapies in multiple myeloma.
Results: Here, we present recommendations for optimizing patient selection, referral pathways, and adverse event management in the Canadian practice setting. These recommendations are relevant for hematologists/oncologists, oncology nurses, pharmacists, nurse practitioners, physician assistants, and other providers who treat patients with multiple myeloma, as well as individuals with multiple myeloma and their care partners. These recommendations will be of interest to clinicians who treat patients with MM at community clinics and hospitals and who may be interested in referring patients for T-cell-redirecting therapy.
Keywords: CAR T-cells; T-cell–redirecting therapy; adverse events; bispecific antibodies; consensus statement; multiple myeloma; referral.
Copyright © 2024 Lancman, Song, White, Crosbie, Sharif, Emond, Saleem Raza, Elias, Kaedbey and Chu.
Conflict of interest statement
MC: Grant support from Celgene and Bristol Myers Squibb and consulting fees and honoraria from Amgen, AstraZeneca, Celgene/Bristol Myers Squibb, Gilead, Janssen and Teva. TC: Honoraria from AbbVie, Apobiologix, AstraZeneca, BeiGene, Forus Therapeutics, Janssen, Novartis, and Seagen. MEm: Honoraria from Janssen, Lundbeck, Novartis, and Roche, and consultancy fees from Genzyme, Gilead, Lundbeck, Janssen, Novartis, Nycomed, and Sanofi-Aventis. RK: Honoraria and advisory board fees from Janssen, Bristol Myers Squibb, and Pfizer. Grant support from Pfizer. GL: Advisory board fees from Janssen. KS: advisory board fees and fees for serving as a trial investigator from Amgen Canada, Bristol-Myers Squibb Canada, and Janssen Biotech, and fees for serving as a trial investigator from Takeda Oncology. DW: Honoraria from Amgen, Antengene, BMS, Janssen, Karyopharm, Sanofi and Takeda. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Côté J, Kotb R, Bergstrom DJ, LeBlanc R, Mian HS, Othman I, et al. . First line treatment of newly diagnosed transplant ineligible multiple myeloma: recommendations from the Canadian myeloma research group consensus guideline consortium. Clin Lymphoma Myeloma Leuk. (2023) 23:340–54. doi: 10.1016/j.clml.2023.01.016 - DOI - PubMed
-
- Myeloma Canada . About Multiple Myeloma: incidence & prevalence in Canada . Available online at: https://myelomaCanada.ca/en/about-multiple-myeloma/what-is-myeloma/incid... (Accessed October 24, 2023).
-
- Canadian Cancer Society . Canadian cancer statistics 2023 (2023). Available online at: https://cancer.ca/en/cancer-information/resources/publications/canadian-... (Accessed December 6, 2023).
-
- Mian H, Reece D, Masih-Khan E, McCurdy A, Kardjadj M, Jimenez-Zepeda VH, et al. . Survival and outcomes of newly diagnosed multiple myeloma patients stratified by transplant status 2007-2018: retrospective analysis from the Canadian myeloma research group database. Clin Lymphoma Myeloma Leuk. (2022) 22:608–17. doi: 10.1016/j.clml.2022.03.002 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

