Impact of tumor-treating fields on the survival of Japanese patients with newly diagnosed glioblastoma: A multicenter, retrospective cohort study
- PMID: 39659832
- PMCID: PMC11629686
- DOI: 10.1093/noajnl/vdae176
Impact of tumor-treating fields on the survival of Japanese patients with newly diagnosed glioblastoma: A multicenter, retrospective cohort study
Abstract
Background: The EF-14 clinical trial demonstrated the safety and efficacy of tumor-treating fields (TTFields) for newly diagnosed glioblastoma. This study aimed to clarify the current status, safety, and efficacy of TTFields in Japanese patients who meet the EF-14 inclusion criteria.
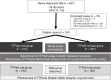
Methods: This was a multicenter retrospective cohort study. Background, treatment, and outcome data of patients who satisfied the inclusion criteria of the EF-14 trial were collected from 45 institutions across Japan. The rate, determinants, and current status of TTField use, including its safety and efficacy in terms of progression and survival, were retrospectively investigated. This study was conducted in accordance with the STROBE checklist.
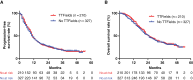
Results: Among the 607 patients enrolled, 70 were excluded due to progressive disease during radiation and temozolomide therapy, age > 80 years old, and Karnofsky Performance Status score of <70. Among the remaining 537 patients, 210 (39%) underwent TTField treatment. Multivariate analysis revealed younger age and spouse as a caregiver as significant factors for TTField use. The compliance rate of TTField use exceeded 75% in 60% of patients, with a median TTField usage duration of 11 months. Skin disorders requiring medical treatment occurred in 56% of patients. Multivariate Cox proportional hazards analysis in the whole series and propensity score-matched analysis revealed that TTField use was not a prognostic factor for progression-free survival (PFS) or overall survival (OS).
Conclusions: TTField use did not have a substantial effect on either PFS or OS in Japanese patients with glioblastoma, despite compliance rates comparable to those observed in the EF-14.
Keywords: Asian population; determinant of use; glioblastoma; survival; tumor-treating fields.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
M.Ka. received honoraria from Novocure and Eisai and research funding from Eisai. K.S. received honoraria from Novocure and Eisai and research funding from Eisai and Nippon Kayaku. S.Y. received honoraria from Novocure. M.N. received honoraria from Novocure and Eisai. T.M. received honoraria from Novocure and Eisai. M.N. received honoraria from Novocure, Eisai, and Nippon Kayaku and research funding from Eisai and MSD. K.Y. received honoraria from Novocure. H.Y. received honoraria from Novocure and Eisai. Y.Nakahara received honoraria from Novocure and Eisai. Y.H. received honoraria from Novocure and Eisai and research funding from Eisai. M.Ki. received honoraria from Novocure and Eisai. A.Y. received research funding from Eisai. Y.K. received honoraria from Novocure and Eisai. M.T. received research funding for clinical trial from Eisai. Y.A. received honoraria from Novocure, Eisai, and Nippon Kayaku. Y.Narita received honoraria from Novocure and Eisai and research funding from Eisai. All remaining authors have declared no conflicts of interest.
Figures

References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Muragaki Y, Akimoto J, Maruyama T, et al. Phase II clinical study on intraoperative photodynamic therapy with talaporfin sodium and semiconductor laser in patients with malignant brain tumors. J Neurosurg. 2013;119(4):845–852. - PubMed
LinkOut - more resources
Full Text Sources
