An advanced chitosan based sponges dressing system with antioxidative, immunoregulation, angiogenesis and neurogenesis for promoting diabetic wound healing
- PMID: 39659839
- PMCID: PMC11629240
- DOI: 10.1016/j.mtbio.2024.101361
An advanced chitosan based sponges dressing system with antioxidative, immunoregulation, angiogenesis and neurogenesis for promoting diabetic wound healing
Abstract
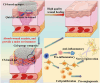
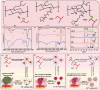
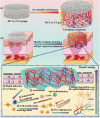
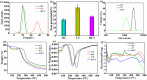
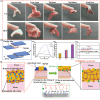
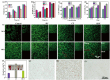
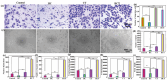
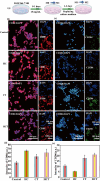
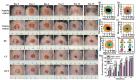
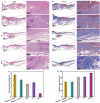
Promoting wound nerve regeneration and synchronously initiating angiogenesis are critical factors in the healing process of diabetic wounds. However, existing research on diabetic wounds mainly focuses on angiogenesis, bacterial infection and reactive oxygen species, often failing to coordinate neurogenesis and angiogenesis. To coordinate the symbiosis of nerves and blood vessels in the diabetic wounds, we successfully designed a multifunctional chitosan (CS)-based sponges by regulating the structure of CS specifically for diabetic wound healing. This sponge, which facilitates effective exudate transfer and modulates the wound microenvironment, was constructed using hydroxybutyl CS grafted with thioctic acid (TA), named as HCT sponge. When applied in a humid environment, the hydrophobic side chains of the HCT sponge interact with self-assembled hydrophobic domains, forming gel-sponge composite. Experimental results showed that the adhesion strength of the HCT sponge to wet porcine skin was 70.3 kPa. Additionally, the sponge exhibited favorable degradability, cytocompatibility and antioxidant properties. As it is shown in the experiments in vitro, sponge can not only promote cell proliferation, migration, and blood vessel formation, but also promote M2 macrophage polarization. Moreover, the rat liver and femoral artery injury model validated that the HCT sponge can effectively treat heavy bleeding from wounds efficacy through quickly sealing wounds and the formation of multiple hemostatic dams. In vivo studies indicated that the HCT sponge significantly accelerated the diabetic wound healing process compared to the recombinant bovine basic fibroblast growth factor gel, achieving a better recovery from the HCT sponge after 15 days. Pathological results show that the designed novel sponge holds considerable promise for treating diabetic wound, allowing regenerative neurogenesis and angiogenesis at the wound site, which provides a significant potential for further improving clinical applications.
Keywords: Angiogenesis; CS-based sponges; Diabetic wound healing; Nerve regeneration; Wet tissue adhesion.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that no conflicts of financial interests or personal relationships have influenced the work reported in this paper.
Figures



References
-
- Jaul E. Non-healing wounds: the geriatric approach. Arch. Gerontol. Geriatr. 2009;49(2):224–226. - PubMed
-
- Xiong Y., Lin Z., Bu P., Yu T., Endo Y., Zhou W., Sun Y., Cao F., Dai G., Hu Y., Lu L., Chen L., Cheng P., Zha K., Shahbazi M.A., Feng Q., Mi B., Liu G. A whole-course-repair system based on neurogenesis-angiogenesis crosstalk and macrophage reprogramming promotes diabetic wound healing. Adv. Mater. 2023;35(19) - PubMed
-
- Butenko S., Nagalla R.R., Guerrero Juarez C.F., Palomba F., David L.M., Nguyen R.Q., Gay D., Almet A.A., Digman M.A., Nie Q., Scumpia P.O., Plikus M.V., Liu W.F. Hydrogel crosslinking modulates macrophages, fibroblasts, and their communication, during wound healing. Nat. Commun. 2024;15(1):6820. - PMC - PubMed
-
- Fan X., Huang J., Zhang W., Su Z., Li J., Wu Z., Zhang P. A multifunctional, tough, stretchable, and transparent curcumin hydrogel with potent antimicrobial, antioxidative, anti-inflammatory, and angiogenesis capabilities for diabetic wound healing. ACS Appl. Mater. Interfaces. 2024;16(8):9749–9767. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

