Optimization of concentrations and exposure durations of commonly used positive controls in the in vitro alkaline comet assay
- PMID: 39659849
- PMCID: PMC11630343
- DOI: 10.1093/toxres/tfae195
Optimization of concentrations and exposure durations of commonly used positive controls in the in vitro alkaline comet assay
Abstract
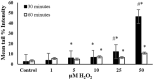
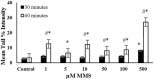
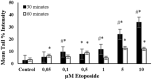
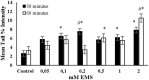
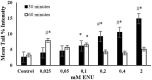
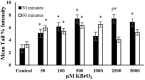
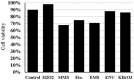
Endogenous and exogenous factors cause DNA damage through chemical changes in the genomic DNA structure. The comet assay is a versatile, rapid, and sensitive method for evaluating DNA integrity at the individual cell level. It is used in human biomonitoring studies, the identification of DNA lesions, and the measurement of DNA repair capacity. Despite its widespread application, variations between studies remain problematic, often due to the lack of a common protocol and appropriate test controls. Using positive controls is essential to assess inter-experimental variability and ensure reliable results. Hydrogen peroxide (H2O2) is the most commonly used positive control, while potassium bromate (KBrO₃), methyl methanesulfonate (MMS), ethyl methanesulfonate (EMS), N-ethyl-N-nitrosourea (ENU), and etoposide are used less frequently. However, differences in concentrations and exposure durations prevent the confirmation of test method efficacy. This study investigates the dose-response relationship for H2O2, KBrO3, MMS, EMS, ENU and etoposide in the comet assay for 30 and 60-minute exposure durations in 3T3 cell lines. Accordingly recommended concentrations and exposure durations were found to be 50 μM 30 minutes (H2O2); 500 μM 60 min. (MMS); 10 μM 30 min. (Etoposide); 0.2 mM 30 min. and 2 mM 60 min. (EMS); 2 mM 30 min. (ENU); 500 μM 30 min. and 50 μM 60 min. (KBrO3). Our findings will contribute to reducing inter-laboratory variability by offering guidance on selecting doses and exposure durations for positive controls in the in vitro alkaline comet assay.
Keywords: Comet assay; Positive controls; Standardization; in vitro.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
-
- Bolognesi C, Cirillo S, Chipman JK. Comet assay in ecogenotoxicology: applications in Mytilus sp. Mutat Res Genet Toxicol Environ Mutagen. 2019:842:50–59. - PubMed
-
- Gajski G, Ravlić S, Godschalk R, Collins A, Dusinska M, Brunborg G. Application of the comet assay for the evaluation of DNA damage in mature sperm. Mutat Res Rev Mutat Res. 2021:788:108398. - PubMed
-
- Møller P. The comet assay: ready for 30 more years. Mutagenesis. 2018:33(1):1–7. - PubMed
LinkOut - more resources
Full Text Sources

