Optimal Dosing Recommendations of Clonidine in Pediatrics Using Physiologically Based Pharmacokinetic Modeling
- PMID: 39659862
- PMCID: PMC11627573
- DOI: 10.5863/1551-6776-29.6.636
Optimal Dosing Recommendations of Clonidine in Pediatrics Using Physiologically Based Pharmacokinetic Modeling
Abstract
Objective: Clonidine has been widely used in the pediatric population to treat neonatal abstinence syndrome (NAS), attention deficit hyperactivity disorder (ADHD), sedation, and Tourette's syndrome; however, there is no consensus on dosing. This research aims to recommend optimal dosing of clonidine in the pediatric population using physiologically based pharmacokinetic (PBPK) modeling.
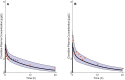
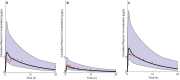
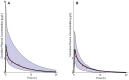
Methods: The pediatric PBPK model was developed from an adult model by scaling the clearance processes from adults to pediatrics using ontogeny equations. The adult and pediatric models were verified using clinical PK data, and the model performance was evaluated based on visual predictive checks and absolute fold error (AFE). The final pediatric PBPK model was used to simulate clonidine PK in the virtual pediatric population. The optimal dose was recommended based on a target concentration representing clonidine's α-2 central agonist activity (EC50 = 40.5 nM).
Results: The adult and pediatric models predicted well, with more than 90% of observed data captured within the 95% prediction interval of simulated data. The AFE values were within 2-fold for clonidine plasma concentrations from observed and predicted data. The pediatric simulations showed that 30 µg/kg dose orally for neonates and 0.9 mg/day orally for children (6-17 years) are optimal for achieving target concentrations for maximal α-2 adrenergic activity.
Conclusions: The pediatric PBPK model of clonidine scaled from the adult PBPK model provided optimal dosing recommendations for clonidine in different pediatric age groups. The pediatric PBPK model described in this study can be extended to other pediatric age groups and routes of administration.
Keywords: CYP ontogeny; Tourette’s syndrome; attention deficit hyperactivity disorder; clonidine; neonatal abstinence syndrome; physiologically based pharmacokinetic (PBPK) modeling; α-2 receptor agonist.
Copyright. Pediatric Pharmacy Association. All rights reserved. For permissions, email: membership@pediatricpharmacy.org.
Conflict of interest statement
Disclosure. CMS is also employed by Differentia Bio; CMS holds no stock. The work was completed by CMS while at WSU, Dayton, Ohio. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Figures
References
-
- Wood RA. The therapeutic uses of clonidine. Scott Med J . 1979;24(3):226–232. - PubMed
-
- Houston MC. Clonidine hydrochloride: review of pharmacologic and clinical aspects. Prog Cardiovasc Dis . 1981;23(5):337–350. - PubMed
-
- Newcorn JH, Krone B, Dittmann RW. Nonstimulant treatments for ADHD. Child Adolesc Psychiatr Clin N Am . 2022;31(3):417–435. - PubMed
LinkOut - more resources
Full Text Sources
