Perspective: Pathological transdifferentiation-a novel therapeutic target for cardiovascular diseases and chronic inflammation
- PMID: 39660114
- PMCID: PMC11628510
- DOI: 10.3389/fcvm.2024.1500775
Perspective: Pathological transdifferentiation-a novel therapeutic target for cardiovascular diseases and chronic inflammation
Abstract
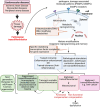
Pathological transdifferentiation, where differentiated cells aberrantly transform into other cell types that exacerbate disease rather than promote healing, represents a novel and significant concept. This perspective discusses its role and potential targeting in cardiovascular diseases and chronic inflammation. Current therapies mainly focus on mitigating early inflammatory response through proinflammatory cytokines and pathways targeting, including corticosteroids, TNF-α inhibitors, IL-1β monoclonal antibodies and blockers, IL-6 blockers, and nonsteroidal anti-inflammatory drugs (NSAIDs), along with modulating innate immune memory (trained immunity). However, these approaches often fail to address long-term tissue damage and functional regeneration. For instance, fibroblasts can transdifferentiate into myofibroblasts in cardiac fibrosis, and endothelial cells may undergo endothelial to mesenchymal transition (EndMT) in vascular remodeling, resulting in fibrosis and impaired tissue function. Targeting pathological transdifferentiation represents a promising therapeutic avenue by focusing on key signaling pathways that drive these aberrant cellular phenotypic and transcriptomic transitions. This approach seeks to inhibit these pathways or modulate cellular plasticity to promote effective tissue regeneration and prevent fibrosis. Such strategies have the potential to address inflammation, cell death, and the resulting tissue damage, providing a more comprehensive and sustainable treatment solution. Future research should focus on understanding the mechanisms behind pathological transdifferentiation, identifying relevant biomarkers and master regulators, and developing novel therapies through preclinical and clinical trials. Integrating these new therapies with existing anti-inflammatory treatments could enhance efficacy and improve patient outcomes. Highlighting pathological transdifferentiation as a therapeutic target could transform treatment paradigms, leading to better management and functional recovery of cardiovascular tissues in diseases and chronic inflammation.
Keywords: cardiovascular diseases; chronic inflammation; endothelial to mesenchymal transition; epithelial to mesenchymal transition; pathological transdifferentiation; vascular smooth muscle to mesenchymal transition.
© 2024 Yang, Ben Issa, Saaoud, Xu, Shao, Lu, Dornas, Cueto, Jiang, Wang and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Lu Y, Sun Y, Xu K, Saaoud F, Shao Y, Drummer C, et al. Aorta in pathologies may function as an immune organ by upregulating secretomes for immune and vascular cell activation, differentiation and trans-differentiation-early secretomes may serve as drivers for trained immunity. Front Immunol. (2022) 13:858256. 10.3389/fimmu.2022.858256 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources