Warehouse-based, immunopeptidome-guided design of personalised peptide vaccines shows feasibility in clinical trial evaluation in CLL patients
- PMID: 39660140
- PMCID: PMC11628388
- DOI: 10.3389/fimmu.2024.1482715
Warehouse-based, immunopeptidome-guided design of personalised peptide vaccines shows feasibility in clinical trial evaluation in CLL patients
Abstract
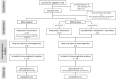
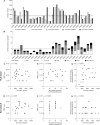
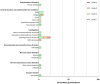
Cancer peptide vaccination represents a promising therapeutic approach, but has been hampered by lack of suitable antigens and restricted applicability due to different HLA backgrounds of individual patients. We here introduce a novel warehouse-based concept for composition of personalized peptide vaccines and report on its successful application in a Phase II clinical trial in patients with chronic lymphocytic leukemia (CLL) after first-line therapy. 26 CLL patients in at least partial remission (PR) after 6 months of immuno-chemotherapy were vaccinated with a personalized vaccine compiled from a premanufactured peptide warehouse comprising immunopeptidome-defined CLL-associated peptides. Primary objective was evaluation of immunogenicity, secondary objectives were safety and minimal residual disease (MRD) response. Immunopeptidome-guided vaccine composition was throughout successful, proving the feasibility of warehouse-based vaccine design. Vaccination was well tolerated, with local injection site reactions being the most common adverse event. Only few patients showed vaccine-induced T cell responses, attributable to their inability to mount strong immune responses due to immune-chemotherapy and lack of potent adjuvant formulations. Both issues are addressed within a follow-up trial (NCT04688385), combining the immunopeptidome-guided warehouse-based vaccine design reported here with a potent novel adjuvant evaluating personalized multi- peptide vaccination in CLL patients under T cell supportive BTK inhibitor therapies.
Clinical trial registration: www.clinicaltrialsregister.eu, identifier NCT02802943.
Keywords: CLL; immunopeptidome; peptide; trial; vaccine; warehouse.
Copyright © 2024 Heitmann, Jung, Wacker, Maringer, Nelde, Bauer, Denk, Hoenisch-Gravel, Richter, Oezbek, Dubbelaar, Bilich, Pumptow, Martus, Illerhaus, Denzlinger, Steinbach, Aulitzky, Müller, Dörfel, Rammensee, Salih and Walz.
Conflict of interest statement
H-GR and JW are listed as inventors on a patent related to the CLL vaccine cocktail. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Cramer P, von Tresckow J, Bahlo J, Robrecht S, Langerbeins P, Al-Sawaf O, et al. . Bendamustine followed by obinutuzumab and venetoclax in chronic lymphocytic leukaemia (CLL2-BAG): primary endpoint analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. (2018) 19:1215–28. doi: 10.1016/S1470-2045(18)30414-5 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

