Cobalt-Based Ferrite Modified Carbon Nanotubes Fibers for Flexible and Disposable Microelectrode Toward Electrochemical Glucose Sensing
- PMID: 39660348
- PMCID: PMC11627181
- DOI: 10.1002/ansa.202400032
Cobalt-Based Ferrite Modified Carbon Nanotubes Fibers for Flexible and Disposable Microelectrode Toward Electrochemical Glucose Sensing
Abstract
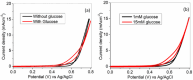
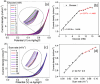
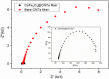
Glucose detection is critical in clinical health and the food industry, particularly in the diagnosis of blood sugar levels. Carbon-based fiber materials have recently featured prominently as non-enzymatic electrochemical glucose detectors. Herein, cobalt-based ferrite (CoFe2O4) in the form of nanoparticles has been successfully fabricated over the carbon nanotubes (CNTs) fiber via a simple hydrothermal process. Fabricated microelectrode (CoFe2O4@CNTs) was investigated as an electrocatalyst toward the non-enzymatic electrochemical glucose sensors. The structure and morphology of the modified fiber were studied by scanning electron microscopy including energy-dispersive X-ray spectroscopy. The electrochemical capability of the microelectrode was analyzed by using different electrochemical techniques including cyclic voltammetry, chronoamperometry, and electrochemical impedance spectroscopy (EIS). The proposed sensors exhibited a superb sensitivity of 0.21 µAcm-2 mM-1, a good linear range from 1 to 9 mM, and a lower detection limit of 1.7 mM. Further investigation via EIS indicated the low charge transfer resistance as compared to the bare CNTs-based fiber. Outcomes revealed that the material can potentially prove promising for the disposable microelectrode toward electrochemical glucose sensing.
© 2024 The Author(s). Analytical Science Advances published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Li M., Fang L., Zhou H., et al., “Three‐Dimensional Porous MXene/NiCo‐LDH Composite for High Performance Non‐Enzymatic Glucose Sensor,” Applied Surface Science 495 (2019): 143554.
-
- Chaianantakul N., Wutthi K., Kamput N., et al., “Development of Mini‐Spectrophotometer for Determination of Plasma Glucose,” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 204 (2018): 670–676. - PubMed
-
- Li Z., Huo P., Gong C., Deng C., and Pu S., “Boric‐Acid‐Modified Fe3O4@PDA@UiO‐66 for Enrichment and Detection of Glucose by Matrix‐Assisted Laser Desorption/Ionization Time‐of‐Flight Mass Spectrometry,” Analytical and Bioanalytical Chemistry 412 (2020): 8083–8092. - PubMed
-
- Chen J., Yan J., Dou B., Feng Q., Miao X., and Wang P., “Aggregatable Thiol‐Functionalized Carbon Dots‐Based Fluorescence Strategy for Highly Sensitive Detection of Glucose Based on Target‐Initiated Catalytic Oxidation,” Sensors and Actuators B: Chemical 330 (2021): 129325.
-
- Wei C., Zou X., Liu Q., Li S., Kang C., and Xiang W., “A Highly Sensitive Non‐Enzymatic Glucose Sensor Based on CuS Nanosheets Modified Cu2O/CuO Nanowire Arrays,” Electrochimica Acta 334 (2020): 135630.
LinkOut - more resources
Full Text Sources
