Effect of Decellularized Amniotic Membrane on the Tendon-Bone Integration in Rotator Cuff Repair: A Comparative Rat Model Study
- PMID: 39660410
- PMCID: PMC11787985
- DOI: 10.1111/os.14316
Effect of Decellularized Amniotic Membrane on the Tendon-Bone Integration in Rotator Cuff Repair: A Comparative Rat Model Study
Abstract
Objective: Rotator cuff retear after arthroscopy repair is a difficult complication that is often due to poor tendon-bone healing. Decellularized amniotic membrane (DAM) has a variety of bioactive substances which have great potential to enhance tendon-bone healing. However, DAM has three layers, of which the middle basement layer is dense and thick. Whether DAM will hinder tendon-bone healing of rotator cuff after surgical repair is unclear. Our study aims to investigate the effect of DAM on tendon-bone healing of the rotator cuff after surgical repair.
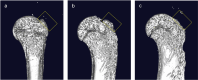
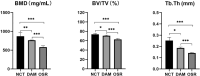
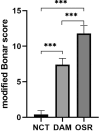
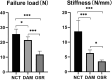
Methods: Thirty-three Sprague-Dawley (SD) rats were selected to establish unilateral supraspinatus (ST) tear models and were randomly treated with only suturing repair (OSR group, n = 11), and suturing repair with DAM placed between the ST and bone (DAM group, n = 11). In the normal control group (NCT group, n = 11), the supraspinatus was only exposed but not detached or repaired. After 4 weeks the rats were sacrificed. The assessment of specimens was conducted by micro-CT analysis, histopathological evaluation, and biomechanical testing.
Results: The DAM group had a significantly higher ultimate load to failure, new bone volume, and histological evaluation at 4 weeks after surgery than the OSR group. When comparing the DAM group to the NCT group, the DAM group performed slightly worse in biomechanical testing, micro-CT analysis, and histological evaluation.
Conclusion: When placed between tendon and bone at the rotator cuff footprint, DAM, despite its dense and thick basement layer, does not impede tendon-bone healing after surgical repair for rotator cuff injury, but rather promotes increased healing quality and biomechanical properties. However, the healing quality and biomechanical properties are still lower than that of the normal rotator cuff, and further improvement should be made to the application strategy of a DAM.
Keywords: decellularized amniotic membrane; retear; rotator cuff injury; surgical repair; tendon–bone healing.
© 2024 The Author(s). Orthopaedic Surgery published by Tianjin Hospital and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Interposition of acellular amniotic membrane at the tendon to bone interface would be better for healing than overlaying above the tendon to bone junction in the repair of rotator cuff injury.Chin J Traumatol. 2025 May;28(3):187-192. doi: 10.1016/j.cjtee.2024.04.001. Epub 2024 Apr 20. Chin J Traumatol. 2025. PMID: 38688817 Free PMC article.
-
Small Subchondral Drill Holes Improve Marrow Stimulation of Rotator Cuff Repair in a Rabbit Model of Chronic Rotator Cuff Tear.Am J Sports Med. 2020 Mar;48(3):706-714. doi: 10.1177/0363546519896350. Epub 2020 Jan 13. Am J Sports Med. 2020. PMID: 31928410
-
Effect of the Interposition of Calcium Phosphate Materials on Tendon-Bone Healing During Repair of Chronic Rotator Cuff Tear.Am J Sports Med. 2014 Aug;42(8):1920-9. doi: 10.1177/0363546514532781. Epub 2014 May 22. Am J Sports Med. 2014. PMID: 24853168
-
Effects of Estrogen-Deficient State on Rotator Cuff Healing.Am J Sports Med. 2019 Feb;47(2):389-397. doi: 10.1177/0363546518815869. Epub 2019 Jan 9. Am J Sports Med. 2019. PMID: 30625277
-
Decellularized Bovine Pericardial Patch Loaded With Mesenchymal Stromal Cells Enhance the Mechanical Strength and Biological Healing of Large-to-Massive Rotator Cuff Tear in a Rat Model.Arthroscopy. 2022 Nov;38(11):2987-3000. doi: 10.1016/j.arthro.2022.06.004. Epub 2022 Jun 16. Arthroscopy. 2022. PMID: 35716989
References
-
- Dang A. and Davies M., “Rotator Cuff Disease: Treatment Options and Considerations,” Sports Medicine and Arthroscopy Review 26, no. 3 (2018): 129–133. - PubMed
-
- Kwon D. R., Jung S. M., Jang J., et al., “A 3‐Dimensional Bioprinted Scaffold With Human Umbilical Cord Blood–Mesenchymal Stem Cells Improves Regeneration of Chronic Full‐Thickness Rotator Cuff Tear in a Rabbit Model,” American Journal of Sports Medicine 48, no. 4 (2020): 947–958. - PubMed
-
- Rossi L. A., Chahla J., Verma N. N., Millett P. J., and Ranalletta M., “Retears of the Rotator Cuff,” JBJS Reviews 8, no. 1 (2020): e0039. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

