Simple and versatile electrochemical synthesis of highly substituted 2,1-benzisoxazoles
- PMID: 39660434
- PMCID: PMC11632592
- DOI: 10.1039/d4ob01875c
Simple and versatile electrochemical synthesis of highly substituted 2,1-benzisoxazoles
Abstract
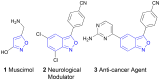
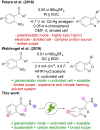
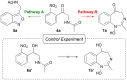
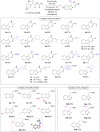
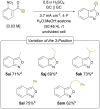
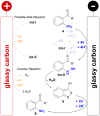
A sustainable, general and scalable electrochemical protocol for direct access to 3-(acylamidoalkyl)-2,1-benzisoxazoles by cathodic reduction of widely accessible nitro arenes is established. The method is characterised by a simple undivided set-up under constant current conditions, inexpensive and reusable carbon-based electrodes, and environmentally benign reaction conditions. The versatility of the developed protocol is demonstrated on 39 highly diverse examples with up to 81% yield. A 50-fold scale-up electrolysis highlights its relevance for preparative applications.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Taylor R. D. MacCoss M. Lawson A. D. G. J. Med. Chem. 2014;57:5845. doi: 10.1021/jm4017625. - DOI - PubMed
- Arya G. C. Kaur K. Jaitak V. Eur. J. Med. Chem. 2021;221:113511. doi: 10.1016/j.ejmech.2021.113511. - DOI - PubMed
- Pandhurnekar C. P. Pandhurnekar H. C. Mungole A. J. Butoliya S. S. Yadao B. G. J. Heterocycl. Chem. 2023;60:537. doi: 10.1002/jhet.4586. - DOI
-
- Zhu X.-Q. He J.-J. Zhou B. Ye L.-W. Cell Rep. Phys. Sci. 2023;4:101645. doi: 10.1016/j.xcrp.2023.101645. - DOI
LinkOut - more resources
Full Text Sources

