Unlocking Nature's Potential: Ferritin as a Universal Nanocarrier for Amplified Cancer Therapy Testing via 3D Microtissues
- PMID: 39660468
- PMCID: PMC11672483
- DOI: 10.1021/acsami.4c12524
Unlocking Nature's Potential: Ferritin as a Universal Nanocarrier for Amplified Cancer Therapy Testing via 3D Microtissues
Abstract
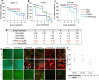
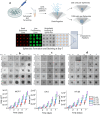
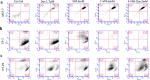
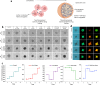
In the existing development of extensive drug screening models, 3D cell cultures outshine conventional 2D monolayer cells by closely imitating the in vivo tumor microenvironment. This makes 3D culture a more physiologically relevant and convenient system in the regime of preclinical drug testing. In the nanomedicinal world, nanoconjugates as nanocarriers are largely hunted due to their capability of precisely binding to target cells and distributing essential dosages of therapeutic drugs with enhanced safety profiles. Thus, for boosted drug availability, the evolution from conventional drug treatment to combination therapies and last switching to drug carriers has gained significant progression in cancer cure. In contrast to conventional engineered nanoparticles, herein, we successfully designed biomolecule (ferritin)-based drug nanoconjugates effective both as a single drug (valproic acid-VPA) and twin-drug (valproic acid/doxorubicin-Dox) carriers, which dramatically enhance the proficiency of the tumor therapeutic modality. To question the reported adjuvant drug property of VPA, we progressed utilizing at first VPA alone as an effective yet exclusive tumor therapy when delivered via some carrier molecule, in particular protein. Subsequently, we paralleled this comprehensive investigation output to compare and test the coloading strategy of drugs and observe the synergistic and/or additive behavior of VPA in conjugation with other anticancer agents (Dox) while given via a carrier molecule. To approach this, VPA and/or Dox molecules were encapsulated into the ferritin (F) cavity using a thermosensitive synthesis method by maintaining the temperature at 60 °C. The successful encapsulation of drugs in the protein nanocage was confirmed through various characterization techniques. The F-VPA/F-VPA-Dox nanoconjugates exhibited similar morphology and structural characteristics to the hollow ferritin cage and showed significant cytotoxicity than the naked drugs when tested on physiologically relevant 3D spheroid models. Precisely, our first designed carrier nanoconjugate, i.e., F-VPA, offered more than a 3-fold increased intratumoral drug concentration than free VPA and significantly suppressed tumor growth after a single-dose treatment. However, our second modeled carrier nanoconjugate, viz. F-VPA-Dox, revealed an extended median survival period and lesser toxicity when administered at a much more effective dose (∼3-5 μM), in 3D tumor spheroid models of various cancer cell lines. All in all, importantly, ferritin nanoconjugates exhibited an enhanced tumor inhibition rate with a single-dose treatment, which further confirms the benefits of the active targeting property of these nanocarriers. Moreover, these nanocarriers also offer to deliver a significant dose of the therapeutic drug into tumor cells, alongside tremendous biocompatibility and safety profiles in numerous tumor 3D spheroid models.
Keywords: 3D microtissues; drug nanoconjugate; ferritin; nanocarrier materials; protein; spheroids.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Riedl A.; Schlederer M.; Pudelko K.; Stadler M.; Walter S.; Unterleuthner D.; Unger C.; Kramer N.; Hengstschläger M.; Kenner L.; et al. Comparison of Cancer Cells Cultured in 2D vs 3D Reveals Differences in AKT/mTOR/S6-Kinase Signaling and Drug Response. J. Cell Sci. 2017, 130 (1), 203–218. 10.1242/jcs.188102. - DOI - PubMed
-
- Fontoura J. C.; Viezzer C.; Dos Santos F. G.; Ligabue R. A.; Weinlich R.; Puga R. D.; Antonow D.; Severino P.; Bonorino C. Comparison of 2D and 3D Cell Culture Models for Cell Growth, Gene Expression and Drug Resistance. Mater. Sci. Eng., C 2020, 107, 110264. 10.1016/j.msec.2019.110264. - DOI - PubMed
-
- Falvo E.; Tremante E.; Arcovito A.; Papi M.; Elad N.; Boffi A.; Morea V.; Conti G.; Toffoli G.; Fracasso G.; Giacomini P.; Ceci P. Improved Doxorubicin Encapsulation and Pharmacokinetics of Ferritin–Fusion Protein Nanocarriers Bearing Proline, Serine, and Alanine Elements. Biomacromolecules 2016, 17 (2), 514–522. 10.1021/acs.biomac.5b01446. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

