Neural responses to stress and alcohol cues in individuals with pain with and without alcohol use disorder
- PMID: 39660770
- PMCID: PMC11632857
- DOI: 10.1111/adb.70010
Neural responses to stress and alcohol cues in individuals with pain with and without alcohol use disorder
Abstract
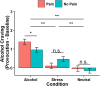
Pain and alcohol use disorder (AUD) frequently co-occur, but the underlying neurobiology is not well-understood. Although many studies have reported disruptions in stress and reward cue-elicited neural reactivity and heightened alcohol craving in individuals with AUD, little is known about these constructs among patients who experience pain. Here, individuals with pain (Pain+, n = 31) and without pain (Pain-, n = 37) completed a well-validated functional magnetic resonance imaging (fMRI) paradigm involving stress (S), alcohol (A) and neutral (N) cue exposure with repeated alcohol craving assessments. Using whole-brain, voxel-based analyses (p < 0.001, whole-brain cluster correction at α < .05), the Pain+ versus Pain- group evidenced greater dorsal anterior cingulate cortex and left amygdala hyperactivation during N, but hypoactivation during the S-N contrast. Additionally, Pain+ exhibited blunted right anterior insular cortex (AIC) during S-N and blunted anteromedial thalamus and left AIC with hyperactive orbitofrontal cortex (OFC) during A-N. Exploratory analyses further revealed that individuals with pain and AUD (n = 17) relative to pain alone (n = 14) showed hyperactive bilateral AIC and hypoactive right dorsal caudate during A-N. Alcohol cue-induced craving, significantly higher in Pain+ (p = 0.03), correlated with blunted right AIC and OFC responses during A-N. In sum, these results provide first evidence of heightened alcohol cue-elicited craving and disrupted stress- and alcohol cue-reactivity within corticostriatal-limbic regions implicated in negative affect and preoccupation/anticipation stages of AUD in those with pain and with comorbid pain and AUD. Future investigations of pain-AUD interaction are needed that include systematic pain assessment and longitudinal designs with larger sample sizes.
Keywords: AUD; alcohol craving; fMRI; insula; pain; stress and alcohol cue reactivity.
© 2024 The Author(s). Addiction Biology published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Conflict of interest statement
All authors declare no financial relationships with commercial interests.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

