Comparison of the molecular FluoroType Mycobacteria VER 1.0 and the Maldi BioTyper Mycobacteria assays for the identification of non-tuberculous mycobacteria
- PMID: 39660810
- PMCID: PMC11784439
- DOI: 10.1128/jcm.01206-24
Comparison of the molecular FluoroType Mycobacteria VER 1.0 and the Maldi BioTyper Mycobacteria assays for the identification of non-tuberculous mycobacteria
Abstract
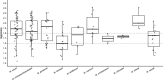
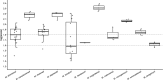
Accurate identification of non-tuberculous mycobacterial (NTM) species is crucial for the diagnosis and appropriate management of NTM infections. This study aimed to evaluate the performance of two assays, FluoroType Mycobacteria VER 1.0 and Maldi BioTyper (MBT) Mycobacteria. The two assays were evaluated using 119 NTM, including 85 slow-growing mycobacteria and 34 rapid-growing mycobacteria, representing a total of 33 species isolated in three French clinical laboratories. We used the GenoType assays as reference method for species identification, followed by 16S rRNA gene sequencing if the GenoType kits returned Mycobacterium sp. Compared to the reference method, the FluoroType Mycobacteria assay provided correct species identification in 89.9% of cases (107/119). Among the most frequently encountered species in clinical settings, low concordance was obtained for Mycobacterium intracellulare (82.4%, 14/17), Mycobacterium gordonae (66.7%, 6/9), and Mycobacterium xenopi (75%, 6/8). Misidentification was obtained in two cases (Mycobacterium smegmatis instead of Mycobacterium mageritense, and Mycobacterium mucogenicum instead of Mycobacterium phocaicum). Using the MBT Mycobacteria assay, 78.1% (93/119) of NTM isolates were correctly identified at the species level. One Mycobacterium europaeum isolate was misidentified as M. intracellulare/Mycobacterium chimaera. In five cases, the assay provided more accurate NTM identification compared to GenoType assays, in which closely related species are identified as a group. The FluoroType Mycobacteria VER 1.0 and the MBT Mycobacteria assays are useful tools for NTM identification from positive cultures, reducing handling time compared to GenoType assays. Their routine use in laboratories must take into consideration their performance and limitations in clinical settings.
Keywords: FluoroType; MALDI-TOF MS; diagnosis; identification; nontuberculous mycobacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. 2020. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis 71:e1–e36. doi:10.1093/cid/ciaa241 - DOI - PMC - PubMed
-
- Lange C, Böttger EC, Cambau E, Griffith DE, Guglielmetti L, van Ingen J, Knight SL, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, Wagner D, Winthrop K, Daley CL, expert panel group for management recommendations in non-tuberculous mycobacterial pulmonary diseases . 2022. Consensus management recommendations for less common non-tuberculous mycobacterial pulmonary diseases. Lancet Infect Dis 22:e178–e190. doi:10.1016/S1473-3099(21)00586-7 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

