The histidine kinase NahK regulates denitrification and nitric oxide accumulation through RsmA in Pseudomonas aeruginosa
- PMID: 39660891
- PMCID: PMC11784011
- DOI: 10.1128/jb.00408-24
The histidine kinase NahK regulates denitrification and nitric oxide accumulation through RsmA in Pseudomonas aeruginosa
Abstract
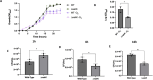
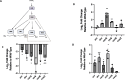
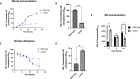
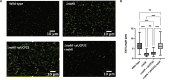
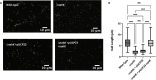
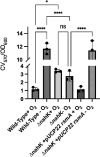
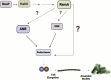
Pseudomonas aeruginosa have a versatile metabolism; they can adapt to many stressors, including limited oxygen and nutrient availability. This versatility is especially important within a biofilm where multiple microenvironments are present. As a facultative anaerobe, P. aeruginosa can survive under anaerobic conditions utilizing denitrification. This process produces nitric oxide (NO) which has been shown to result in cell elongation. However, the molecular mechanism underlying this phenotype is poorly understood. Our laboratory has previously shown that NosP is a NO-sensitive hemoprotein that works with the histidine kinase NahK to regulate biofilm formation in P. aeruginosa. In this study, we identify NahK as a novel regulator of denitrification under anaerobic conditions. Under anaerobic conditions, deletion of nahK leads to a reduction of growth coupled with reduced transcriptional expression and activity of the denitrification reductases. Furthermore, during stationary phase under anaerobic conditions, ΔnahK does not exhibit cell elongation, which is characteristic of P. aeruginosa. We determine the loss of cell elongation is due to changes in NO accumulation in ΔnahK. We further provide evidence that NahK may regulate denitrification through modification of RsmA levels.
Importance: Pseudomonas aeruginosa is an opportunistic multi-drug resistance pathogen that is associated with hospital-acquired infections. P. aeruginosa is highly virulent, in part due to its versatile metabolism and ability to form biofilms. Therefore, better understanding of the molecular mechanisms that regulate these processes should lead to new therapeutics to treat P. aeruginosa infections. The histidine kinase NahK has been previously shown to be involved in both nitric oxide (NO) signaling and quorum sensing through RsmA. The data presented here demonstrate that NahK is responsive to NO produced during denitrification to regulate cell morphology. Understanding the role of NahK in metabolism under anaerobic conditions has larger implications in determining its role in a heterogeneous metabolic environment such as a biofilm.
Keywords: NahK; NosP; RsmA; biofilm; cell elongation; denitrification; nitric oxide; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
The histidine kinase NahK in Pseudomonas aeruginosa is essential for nitric oxide-stress resistance in nutrient starved media.bioRxiv [Preprint]. 2025 Jul 3:2025.07.03.662950. doi: 10.1101/2025.07.03.662950. bioRxiv. 2025. PMID: 40631272 Free PMC article. Preprint.
-
Pseudomonas aeruginosa Dnr-regulated denitrification in microoxic conditions.Microbiol Spectr. 2025 Aug 7:e0068225. doi: 10.1128/spectrum.00682-25. Online ahead of print. Microbiol Spectr. 2025. PMID: 40772714
-
NosP Detection of Heme Modulates Burkholderia thailandensis Biofilm Formation.Biochemistry. 2023 Aug 15;62(16):2426-2441. doi: 10.1021/acs.biochem.3c00187. Epub 2023 Jul 27. Biochemistry. 2023. PMID: 37498555 Free PMC article.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan L, Liang H, Song X, Wu M. 2022. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther 7:199. doi:10.1038/s41392-022-01056-1 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

