Underappreciated layers of antibody-mediated immune synapse architecture and dynamics
- PMID: 39660921
- PMCID: PMC11708040
- DOI: 10.1128/mbio.01900-24
Underappreciated layers of antibody-mediated immune synapse architecture and dynamics
Abstract
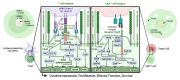
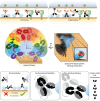
The biologic activities of antibody drugs are dictated by structure-function relationships-emerging from the kind, composition, and degree of interactions with a target antigen and with soluble and cellular antibody receptors of the innate immune system. These activities are canonically understood to be both modular: antigen recognition is driven by the heterodimeric antigen-binding fragment, and innate immune recruitment by the homodimeric constant/crystallizable fragment. The model that treats these domains with a high degree of independence has served the field well but is not without limitations. Here, we consider how new insights, particularly from structural studies, complicate the model of neat biophysical separation between these domains and shape our understanding of antibody effector functions. The emerging model endeavors to explain the phenotypic impact of both antibody intrinsic characteristics and extrinsic features-fitting them within a spatiotemporal paradigm that better accounts for observed antibody activities. In this review, we will use insights from recent models of classical complement complexes and T cell immune synapse formation to explore how structural differences in antibody-mediated immune synapses may relate to their functional diversity.
Keywords: antibody; effector function; immune synapse; mechanism of action.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Aberrant Immunological Synapses Driven by Leukemic Antigen-Presenting Cells.Methods Mol Biol. 2017;1584:533-544. doi: 10.1007/978-1-4939-6881-7_33. Methods Mol Biol. 2017. PMID: 28255724
-
Molecular mechanisms and functional implications of polarized actin remodeling at the T cell immunological synapse.Cell Mol Life Sci. 2015 Feb;72(3):537-556. doi: 10.1007/s00018-014-1760-7. Epub 2014 Oct 30. Cell Mol Life Sci. 2015. PMID: 25355055 Free PMC article. Review.
-
Diversity in immunological synapse structure.Immunology. 2010 Dec;131(4):466-72. doi: 10.1111/j.1365-2567.2010.03366.x. Epub 2010 Oct 29. Immunology. 2010. PMID: 21039474 Free PMC article. Review.
-
From tango to quadrilla: current views of the immunological synapse.Cell Adh Migr. 2007 Jan-Mar;1(1):7-12. Epub 2007 Jan 7. Cell Adh Migr. 2007. PMID: 19262090 Free PMC article. Review.
-
The dendritic cell side of the immunological synapse.Biomol Concepts. 2016 Feb;7(1):17-28. doi: 10.1515/bmc-2015-0028. Biomol Concepts. 2016. PMID: 26741354 Review.
References
-
- Wang P, Gajjar MR, Yu J, Padte NN, Gettie A, Blanchard JL, Russell-Lodrigue K, Liao LE, Perelson AS, Huang Y, Ho DD. 2020. Quantifying the contribution of Fc-mediated effector functions to the antiviral activity of anti-HIV-1 IgG1 antibodies in vivo. Proc Natl Acad Sci U S A 117:18002–18009. doi:10.1073/pnas.2008190117 - DOI - PMC - PubMed
-
- Asokan M, Dias J, Liu C, Maximova A, Ernste K, Pegu A, McKee K, Shi W, Chen X, Almasri C, et al. . 2020. Fc-mediated effector function contributes to the in vivo antiviral effect of an HIV neutralizing antibody. Proc Natl Acad Sci U S A 117:18754–18763. doi:10.1073/pnas.2008236117 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01AI162242/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- U19 AI145825/AI/NIAID NIH HHS/United States
- R01 AI129801/AI/NIAID NIH HHS/United States
- U19AI145825/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI176646/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

