The Global Kidney Patient Trials Network and the CAPTIVATE Platform Clinical Trial Design: A Trial Protocol
- PMID: 39661388
- PMCID: PMC11635535
- DOI: 10.1001/jamanetworkopen.2024.49998
The Global Kidney Patient Trials Network and the CAPTIVATE Platform Clinical Trial Design: A Trial Protocol
Abstract
Importance: Chronic kidney disease (CKD) is a global health priority affecting almost 1 billion people. New therapeutic options and clinical trial innovations such as adaptive platform trials provide an opportunity to efficiently test combination therapies.
Objective: To describe the design and baseline results of the Global Kidney Patient Trials Network (GKPTN) and the design and structure of the global adaptive platform clinical trial Chronic Kidney Disease Adaptive Platform Trial Investigating Various Agents for Therapeutic Effect (CAPTIVATE) to find new therapeutic options and treatments for people with kidney disease.
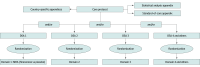
Design, setting, and participants: The GKPTN is a multicenter registry that started in May 2020 and is ongoing, while CAPTIVATE is a multicenter, multifactorial, phase 3, placebo-controlled adaptive platform randomized clinical trial that includes patients with CKD. The first participant was randomized in September 2024. The GKPTN recruits patients from kidney and endocrinology practices, and CAPTIVATE aims to recruit patients from GKPTN sites where possible. Both the GKPTN and CAPTIVATE recruit patients with nondialysis CKD.
Intervention: CAPTIVATE will test several investigational agents or combinations of agents, beginning with a mineralocorticoid receptor antagonist.
Main outcomes and measures: The GKPTN monitors clinical characteristics, treatment, and outcomes to identify eligible clinical trial participants and provide a contemporary global picture of patients with CKD. The primary outcome of CAPTIVATE is to identify investigational agents or combinations of agents to reduce the rate of chronic estimated glomerular filtration rate (eGFR) decline. The default maximum sample size per treatment arm in each domain, based on bayesian simulations, is 500 participants, providing approximately 90% power to detect a clinically meaningful improvement of 2.6 mL/min/1.73 m2 in eGFR at the end of the 104-week study period.
Results: The GKPTN has enrolled 4334 patients across 119 sites in 8 countries (US, Australia, Argentina, China, Italy, Canada, Spain, and Japan). The mean (SD) participant age at enrollment was 64.5 (16.2) years, 2542 participants (58.7%) were female, and diabetic kidney disease was most frequently reported among patients for CKD etiology (1875 [43.3%]). Among the participants, the mean (SD) eGFR was 52.9 (29.3) mL/min/1.73 m2, and the median urinary albumin-to-creatinine ratio was 89 mg/g (coefficient of variation, 20-420 mg/g). In the GKPTN cohort, the mean eGFR decline was steeper among participants with a baseline eGFR of 60 mL/min/1.73 m2 or more (-2.29 [95% CI, -3.14 to -1.44]) compared with those with an eGFR of less than 60 mL/min/1.73 m2 (-1.16 [95% CI, -1.77 to -1.44]) and was progressively steeper in more severe albuminuria subgroups.
Conclusions and relevance: The GKPTN registry and the CAPTIVATE trial have the potential to expand and optimize therapeutic options for people with CKD using an adaptive platform clinical trial design.
Trial registration: ClinicalTrials.gov Identifiers: NCT04389827 and NCT06058585.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

