Synergistic label-free fluorescence imaging and miRNA studies reveal dynamic human neuron-glial metabolic interactions following injury
- PMID: 39661671
- PMCID: PMC11633737
- DOI: 10.1126/sciadv.adp1980
Synergistic label-free fluorescence imaging and miRNA studies reveal dynamic human neuron-glial metabolic interactions following injury
Abstract
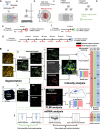
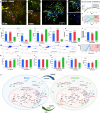
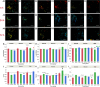
Neuron-glial cell interactions following traumatic brain injury (TBI) determine the propagation of damage and long-term neurodegeneration. Spatiotemporally heterogeneous cytosolic and mitochondrial metabolic pathways are involved, leading to challenges in developing effective diagnostics and treatments. An engineered three-dimensional brain tissue model comprising human neurons, astrocytes, and microglia is used in combination with label-free, two-photon imaging and microRNA studies to characterize metabolic interactions between glial and neuronal cells over 72 hours following impact injury. We interpret multiparametric, quantitative, optical metabolic assessments in the context of microRNA gene set analysis and identify distinct metabolic changes in neurons and glial cells. Glycolysis, nicotinamide adenine dinucleotide phosphate (reduced form) and glutathione synthesis, fatty acid synthesis, and oxidation are mobilized within glial cells to mitigate the impacts of initial enhancements in oxidative phosphorylation and fatty acid oxidation within neurons, which lack robust antioxidant defenses. This platform enables enhanced understanding of mechanisms that may be targeted to improve TBI diagnosis and treatment.
Figures



References
-
- Ismail H., Shakkour Z., Tabet M., Abdelhady S., Kobaisi A., Abedi R., Nasrallah L., Pintus G., Al-Dhaheri Y., Mondello S., El-Khoury R., Eid A. H., Kobeissy F., Salameh J. S., Traumatic brain injury: Oxidative stress and novel anti-oxidants such as mitoquinone and edaravone. Antioxidants 9, 943 (2020). - PMC - PubMed
-
- Barkhoudarian G., Hovda D. A., Giza C. C., The molecular pathophysiology of concussive brain injury—An update. Phys. Med. Rehabil. Clin. N. Am. 27, 373–393 (2016). - PubMed
-
- Wilde E. A., Wanner I.-B., Kenney K., Gill J., Stone J. R., Disner S., Schnakers C., Meyer R., Prager E. M., Haas M., Jeromin A., A framework to advance biomarker development in the diagnosis, outcome prediction, and treatment of traumatic brain injury. J. Neurotrauma 39, 436–457 (2022). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

