High-precision chemical quantum sensing in flowing monodisperse microdroplets
- PMID: 39661672
- PMCID: PMC11633744
- DOI: 10.1126/sciadv.adp4033
High-precision chemical quantum sensing in flowing monodisperse microdroplets
Abstract
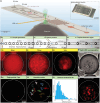
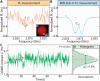
A method is presented for high-precision chemical detection that integrates quantum sensing with droplet microfluidics. Using nanodiamonds (ND) with fluorescent nitrogen-vacancy (NV) centers as quantum sensors, rapidly flowing microdroplets containing analyte molecules are analyzed. A noise-suppressed mode of optically detected magnetic resonance is enabled by pairing controllable flow with microwave control of NV electronic spins, to detect analyte-induced signals of a few hundredths of a percent of the ND fluorescence. Using this method, paramagnetic ions in droplets are detected with low limit-of-detection using small analyte volumes, with exceptional measurement stability over >103 s. In addition, these droplets are used as microconfinement chambers by co-encapsulating ND quantum sensors with various analytes such as single cells, suggesting wide-ranging applications including single-cell metabolomics and real-time intracellular measurements from bioreactors. Important advances are enabled by this work, including portable chemical testing devices, amplification-free chemical assays, and chemical imaging tools for probing reactions within microenvironments.
Figures



References
-
- Degen C. L., Reinhard F., Cappellaro P., Quantum sensing. Rev. Mod. Phys. 89, 035002 (2017).
-
- Zhang T., Pramanik G., Zhang K., Gulka M., Wang L., Jing J., Xu F., Li Z., Wei Q., Cigler P., Chu Z., Toward quantitative biosensing with nitrogen–vacancy center in diamond. ACS Sens. 6, 2077–2107 (2021). - PubMed
-
- Doherty M. W., Dolde F., Fedder H., Jelezko F., Wrachtrup J., Manson N. B., Hollenberg L. C. L., Theory of the ground-state spin of the NV center in diamond. Phys. Rev. B 85, 205203 (2012).
-
- Jelezko F., Wrachtrup J., Single defect centres in diamond: A review. Phys. Status Solidi A Appl. Mater. Sci. 203, 3207–3225 (2006).
-
- Neumann P., Jakobi I., Dolde F., Burk C., Reuter R., Waldherr G., Honert J., Wolf T., Brunner A., Shim J. H., Suter D., Sumiya H., Isoya J., Wrachtrup J., High-precision nanoscale temperature sensing using single defects in diamond. Nano Lett. 13, 2738–2742 (2013). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

