Nucleo-cytoplasmic environment modulates spatiotemporal p53 phase separation
- PMID: 39661689
- PMCID: PMC11633762
- DOI: 10.1126/sciadv.ads0427
Nucleo-cytoplasmic environment modulates spatiotemporal p53 phase separation
Abstract
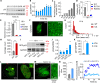
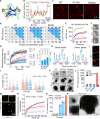
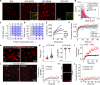
Liquid-liquid phase separation of various transcription factors into biomolecular condensates plays an essential role in gene regulation. Here, using cellular models and in vitro studies, we show the spatiotemporal formation and material properties of p53 condensates that might dictate its function. In particular, p53 forms liquid-like condensates in the nucleus of cells, which can bind to DNA and perform transcriptional activity. However, cancer-associated mutations promote misfolding and partially rigidify the p53 condensates with impaired DNA binding ability. Irrespective of wild-type and mutant forms, the partitioning of p53 into cytoplasm leads to the condensate formation, which subsequently undergoes rapid solidification. In vitro studies show that abundant nuclear components such as RNA and nonspecific DNA promote multicomponent phase separation of the p53 core domain and maintain their liquid-like property, whereas specific DNA promotes its dissolution into tetrameric functional p53. This work provides mechanistic insights into how the life cycle and DNA binding properties of p53 might be regulated by phase separation.
Figures




References
-
- Luzio J. P., Pryor P. R., Bright N. A., Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 8, 622–632 (2007). - PubMed
-
- Boisvert F.-M., van Koningsbruggen S., Navascués J., Lamond A. I., The multifunctional nucleolus. Nat. Rev. Mol. Cell Biol. 8, 574–585 (2007). - PubMed
-
- Shin Y., Brangwynne C. P., Liquid phase condensation in cell physiology and disease. Science 357, 1253 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

