Development of a fibrin-targeted theranostic for gastric cancer
- PMID: 39661705
- PMCID: PMC12087570
- DOI: 10.1126/scitranslmed.adn7218
Development of a fibrin-targeted theranostic for gastric cancer
Abstract
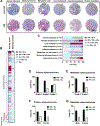
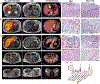
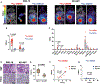
Patients with advanced gastric cancer (GCa) have limited treatment options, and alternative treatment approaches are necessary to improve their clinical outcomes. Because fibrin is abundant in gastric tumors but not in healthy tissues, we hypothesized that fibrin could be used as a high-concentration depot for a high-energy beta-emitting cytotoxic radiopharmaceutical delivered to tumor cells. We showed that fibrin is present in 64 to 75% of primary gastric tumors and 50 to 100% of metastatic gastric adenocarcinoma cores. First-in-human 64Cu-FBP8 fibrin-targeted positron emission tomography (PET) imaging in seven patients with gastric or gastroesophageal junction cancer showed high probe uptake in all target lesions with tumor-to-background (muscle) uptake ratios of 9.9 ± 6.6 in primary (n = 7) and 11.2 ± 6.6 in metastatic (n = 45) tumors. Using two mouse models of human GCa, one fibrin-high (SNU-16) and one fibrin-low (NCI-N87), we showed that PET imaging with a related fibrin-specific peptide, CM500, labeled with copper-64 (64Cu-CM500) specifically bound to and precisely quantified tumor fibrin in both models. We then labeled the fibrin-specific peptide CM600 with yttrium-90 and showed that 90Y-CM600 effectively decreased tumor growth in these mouse models. Mice carrying fibrin-high SNU-16 tumors experienced tumor growth inhibition and prolonged survival in response to either a single high dosage or fractionated lower dosage of 90Y-CM600, whereas mice carrying fibrin-low NCI-N87 tumors experienced prolonged survival in response to a fractionated lower dosage of 90Y-CM600. These results lay the foundation for a fibrin-targeted theranostic that may expand options for patients with advanced GCa.
Conflict of interest statement
P.C. is an inventor on US patent 9,200,017 “Multimodal imaging of fibrin” and WO/2021/081430 application “Fibrin binding compounds for imaging and treatment.” P.C. has equity in and is a consultant to Collagen Medical LLC, has equity in Reveal Pharmaceuticals Inc., has equity in Factor 1A LLC, and has research support from Transcode Therapeutics and Pliant Therapeutics. S.A.E. has research support from Sofie Biosciences, Novartis, and Telix Pharmaceuticals and has received consultation fees from Telix. S.J.K. has served in a consultant/advisory role for Bristol Myers Squibb, Merck, Eli Lilly, Astellas, Daiichi-Sankyo, Pieris, Natera, Novartis, AstraZeneca, Mersana, Sanofi-Aventis, Servier, and Coherus. S.J.K. reports stock/equity in Turning Point Therapeutics and Nuvalent. U.M. is a cofounder, shareholder, and consultant (Scientific Advisory Board) of CytoSite BioPharma. C.A.T. has received research support from Genentech. P.H. serves on the advisory board of Telix. M.R.S. has accepted advisory/consulting fees from Bristol Myers Squibb and Sedqwick Claims Consulting. The other authors declare that they have no competing interests.
Figures



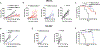
References
-
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F, Cancer statistics for the year 2020: An overview. Int. J. Cancer 149, 778–789 (2021). - PubMed
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 71, 209–249 (2021). - PubMed
-
- Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M, Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 72, 37–41 (1993). - PubMed
-
- Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, Fleig WE, Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev 8, CD004064 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

