Control of Cellular Differentiation Trajectories for Cancer Reversion
- PMID: 39661721
- PMCID: PMC11744559
- DOI: 10.1002/advs.202402132
Control of Cellular Differentiation Trajectories for Cancer Reversion
Abstract
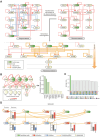
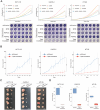
Cellular differentiation is controlled by intricate layers of gene regulation, involving the modulation of gene expression by various transcriptional regulators. Due to the complexity of gene regulation, identifying master regulators across the differentiation trajectory has been a longstanding challenge. To tackle this problem, a computational framework, single-cell Boolean network inference and control (BENEIN), is presented. Applying BENEIN to human large intestinal single-cell transcriptome data, MYB, HDAC2, and FOXA2 are identified as the master regulators whose inhibition induces enterocyte differentiation. It is found that simultaneous knockdown of these master regulators can revert colorectal cancer cells into normal-like enterocytes by synergistically inducing differentiation and suppressing malignancy, which is validated by in vitro and in vivo experiments.
Keywords: Boolean gene regulatory network model; cancer reversion; cell fate control; network reconstruction; single‐cell transcriptome; systems biology.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
K.‐H.C. is an inventor of patents licensed to, board member of and equity owner of biorevert, Inc. No disclosures were reported by the other authors.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
