NAD+ metabolism restriction boosts high-dose melphalan efficacy in patients with multiple myeloma
- PMID: 39661983
- PMCID: PMC11909440
- DOI: 10.1182/bloodadvances.2024013425
NAD+ metabolism restriction boosts high-dose melphalan efficacy in patients with multiple myeloma
Abstract
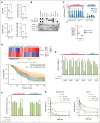
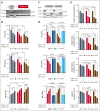
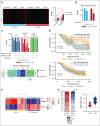
Elevated levels of the NAD+-generating enzyme nicotinamide phosphoribosyltransferase (NAMPT) are a common feature across numerous cancer types. Accordingly, we previously reported pervasive NAD+ dysregulation in multiple myeloma (MM) cells in association with upregulated NAMPT expression. Unfortunately, albeit being effective in preclinical models of cancer, NAMPT inhibition has proven ineffective in clinical trials because of the existence of alternative NAD+ production routes using NAD+ precursors other than nicotinamide. Here, by leveraging mathematical modeling approaches integrated with transcriptome data, we defined the specific NAD+ landscape of MM cells and established that the Preiss-Handler pathway for NAD+ biosynthesis, which uses nicotinic acid as a precursor, supports NAD+ synthesis in MM cells via its key enzyme nicotinate phosphoribosyltransferase (NAPRT). Accordingly, we found that NAPRT confers resistance to NAD+-depleting agents. Transcriptomic, metabolic, and bioenergetic profiling of NAPRT-knockout (KO) MM cells showed these to have weakened endogenous antioxidant defenses, increased propensity to oxidative stress, and enhanced genomic instability. Concomitant NAMPT inhibition further compounded the effects of NAPRT-KO, effectively sensitizing MM cells to the chemotherapeutic drug, melphalan; NAPRT added-back fully rescues these phenotypes. Overall, our results propose comprehensive NAD+ biosynthesis inhibition, through simultaneously targeting NAMPT and NAPRT, as a promising strategy to be tested in randomized clinical trials involving transplant-eligible patients with MM, especially those with more aggressive disease.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures





References
-
- Langseth ØO, Myklebust T, Johannesen TB, Hjertner Ø, Waage A. Incidence and survival of multiple myeloma: a population-based study of 10 524 patients diagnosed 1982-2017. Br J Haematol. 2020;191(3):418–425. - PubMed
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046–1060. - PubMed
-
- Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Nat Rev Dis Primers. 2017;3(1) - PubMed
-
- van de Donk NWCJ, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021;397(10272):410–427. - PubMed
-
- van de Donk NWCJ, Zweegman S. T-cell-engaging bispecific antibodies in cancer. Lancet. 2023;402(10396):142–158. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

