ISPAD Clinical Practice Consensus Guidelines 2024: Screening, Staging, and Strategies to Preserve Beta-Cell Function in Children and Adolescents with Type 1 Diabetes
- PMID: 39662065
- PMCID: PMC11854978
- DOI: 10.1159/000543035
ISPAD Clinical Practice Consensus Guidelines 2024: Screening, Staging, and Strategies to Preserve Beta-Cell Function in Children and Adolescents with Type 1 Diabetes
Abstract
The International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines represent a rich repository that serves as the only comprehensive set of clinical recommendations for children, adolescents, and young adults living with diabetes worldwide. This guideline serves as an update to the 2022 ISPAD consensus guideline on staging for type 1 diabetes (T1D). Key additions include an evidence-based summary of recommendations for screening for risk of T1D and monitoring those with early-stage T1D. In addition, a review of clinical trials designed to delay progression to Stage 3 T1D and efforts seeking to preserve beta-cell function in those with Stage 3 T1D are included. Lastly, opportunities and challenges associated with the recent US Food and Drug Administration (FDA) approval of teplizumab as an immunotherapy to delay progression are discussed. The International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines represent a rich repository that serves as the only comprehensive set of clinical recommendations for children, adolescents, and young adults living with diabetes worldwide. This guideline serves as an update to the 2022 ISPAD consensus guideline on staging for type 1 diabetes (T1D). Key additions include an evidence-based summary of recommendations for screening for risk of T1D and monitoring those with early-stage T1D. In addition, a review of clinical trials designed to delay progression to Stage 3 T1D and efforts seeking to preserve beta-cell function in those with Stage 3 T1D are included. Lastly, opportunities and challenges associated with the recent US Food and Drug Administration (FDA) approval of teplizumab as an immunotherapy to delay progression are discussed.
Keywords: Beta cell; Children; Preservation; Screening; Stages; Type 1 diabetes.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
M.J.H. – Scientific Advisory Board: SAB BIO; consultant: Sanofi and Mannkind. C.S. – participation on an Immunology Advisory Board for Vertex Pharmaceuticals. R.E.J.B. – independent consultant/advisor to Prevention Bio. A.-G.Z. – Advisory Board: Provention Bio/Sanofi; DMC for Provention Bio/Sanofi, Sanofi, and ITB-MED. K.J.B., K.C., J.C., M.E.C., H.E.L., M.T., L.J., F.U., K.L., T.O., M.L.M., D.K.W., and M.L.M.: no conflicts of interest to declare.
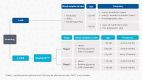
Figures

References
-
- Bingley PJ, Boulware DC, Krischer JP; Type 1 Diabetes TrialNet Study Group . The implications of autoantibodies to a single islet antigen in relatives with normal glucose tolerance: development of other autoantibodies and progression to type 1 diabetes. Diabetologia. 2016;59(3):542–9. - PMC - PubMed
-
- Allen C, Palta M, D’Alessio DJ. Risk of diabetes in siblings and other relatives of IDDM subjects. Diabetes. 1991;40(7):831–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

