Long-term hypoxic atmosphere enhances the stemness, immunoregulatory functions, and therapeutic application of human umbilical cord mesenchymal stem cells
- PMID: 39662502
- PMCID: PMC11634399
- DOI: 10.1302/2046-3758.1312.BJR-2024-0136.R2
Long-term hypoxic atmosphere enhances the stemness, immunoregulatory functions, and therapeutic application of human umbilical cord mesenchymal stem cells
Abstract
Aims: Mesenchymal stem cells (MSCs) are usually cultured in a normoxic atmosphere (21%) in vitro, while the oxygen concentrations in human tissues and organs are 1% to 10% when the cells are transplanted in vivo. However, the impact of hypoxia on MSCs has not been deeply studied, especially its translational application.
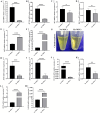
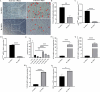
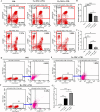
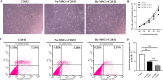
Methods: In the present study, we investigated the characterizations of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) in hypoxic (1%) and normoxic (21%) atmospheres with a long-term culture from primary to 30 generations, respectively. The comparison between both atmospheres systematically analyzed the biological functions of MSCs, mainly including stemness maintenance, immune regulation, and resistance to chondrocyte apoptosis, and studied their joint function and anti-inflammatory effects in osteoarthritis (OA) rats constructed by collagenase II.
Results: We observed that long-term hypoxic culture surpassed normoxic atmosphere during hUC-MSCs culture in respect of promoting proliferation, anti-tumorigenicity, maintaining normal karyotype and stemness, inhibiting senescence, and improving immunoregulatory function and the role of anti-apoptosis in chondrocytes. Furthermore, we demonstrated that the transplantation of long-term hypoxic hUC-MSCs (Hy-MSCs) had a better therapeutic effect on OA rats compared with the hUC-MSCs cultured in the normoxic atmosphere (No-MSCs) in terms of the improved function and swelling recovery in the joints, and substantially inhibited the secretion of pro-inflammatory factors, which effectively alleviated cartilage damage by reducing the expression of matrix metallopeptidase 13 (MMP-13).
Conclusion: Our results demonstrate that Hy-MSCs possess immense potential for clinical applications via promoting stemness maintenance and enhancing immunoregulatory function.
© 2024 Huang et al.
Conflict of interest statement
The authors report grants from the National Key Research and Development Program of China (No. 2022YFA1104300), the National Nature Science Foundation of China (No. 82270302, 82260173, 81970256), the Key Research Project (No. 20192BBH80015, 20202BBG73028), and the Project for Leading Talent of department of science and technology, Jiangxi Province (No. 20204BCJ22035, 20212BDH81020), all related to this study.
Figures



References
-
- Prasopthum A, Cooper M, Shakesheff KM, Yang J. Three-dimensional printed scaffolds with controlled micro-/nanoporous surface topography direct chondrogenic and osteogenic differentiation of mesenchymal stem cells. ACS Appl Mater Interfaces. 2019;11(21):18896–18906. doi: 10.1021/acsami.9b01472. - DOI - PubMed
LinkOut - more resources
Full Text Sources

