A Rare Case of Autoimmune-Mediated Lecithin:Cholesterol Acyltransferase Insufficiency Manifesting as the Acute Onset of Extremely Hypo-High-Density Lipoprotein-Cholesterolemia and Spontaneous Improvement: A Case Report with a Review of the Literature
- PMID: 39662947
- PMCID: PMC12055513
- DOI: 10.5551/jat.65298
A Rare Case of Autoimmune-Mediated Lecithin:Cholesterol Acyltransferase Insufficiency Manifesting as the Acute Onset of Extremely Hypo-High-Density Lipoprotein-Cholesterolemia and Spontaneous Improvement: A Case Report with a Review of the Literature
Abstract
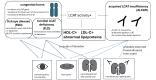
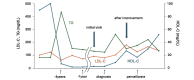
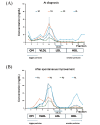
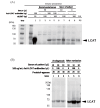
A 59-year-old Japanese woman was referred for an extremely low level of circulating high-density lipoprotein cholesterol (HDL-C). The serum HDL-C level had long been within the normal range but suddenly decreased asymptomatically to 7 mg/dL. She had no typical symptoms associated with familial lecithin, cholesterol acyltransferase deficiency (FLD), including proteinuria, anemia, and corneal opacity. The circulating level of ApoA-1 was also markedly decreased at 48 mg/dL, and the proportion of esterified cholesterol to free cholesterol was irregularly low at 26%. Whole-genome sequencing revealed no apparent pathological mutations in the LCAT gene. Notably, anti-LCAT antibodies were detected in the serum at 146±1.7 ng/mL, resulting in her being diagnosed with acquired LCAT insufficiency (ALCATI) caused by anti-LCAT antibodies. Five years after her HDL-C levels spontaneously decreased, they increased without any identifiable cause. To our knowledge, only six cases of ALCATI caused by anti-LCAT antibodies have been reported to date. In contrast to the present case, previously reported cases of ALCATI manifested proteinuria that improved with steroid therapy. The unique clinical course in the present case highlights the heterogeneity of ALCATI, warranting further research to clarify the molecular pathophysiology of FLD and ALCATI.
Keywords: Autoantibody; Hypo-high-density cholesterolemia; Lecithin-cholesterol acyltransferase; Spontaneous improvement.
Conflict of interest statement
Atsuko Tamaki, Ken Yonaha, Yohei Ishiki, Moriyuki Uehara, Yoshiro Nakayama, Ken-ichiro Honma, Rei Chinen, Tsugumi Uema, Shiki Okamoto, Junko Miyoshi, Mika Kirinashizawa, Kazuki Sato, Tsutomu Aohara, Misato Yamamoto, and Hiroaki Masuzaki have not been disclosed. Masayuki Kuroda received patent royalty and licensing fees from CellGenTech, Inc. Yoshiro Maezawa received clinical research funding from NTT Docomo, Japan. Koutaro Yokote has received honoraria from MSD K.K., Kowa Company, Ltd., Sanofi K.K., Sumitomo Pharma Co., Ltd., Daichi Sankyo Company, Limited, Taisho Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Boehringer Ingelheim International GmbH., Novartis Pharma K.K., Novo Nordisk Pharma Ltd., Bayer Yakuhin, Ltd., Pfizer Japan Inc., and received clinical research funding from CellGenTech, Inc. KY also received scholarship grants from Abbot Japan LLC, Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Kowa Company, Ltd., Sumitomo Pharma Co., Ltd., Taisho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Mitsubishi Tanabe Pharma Corporation, TEIJIN PHARMA LIMITED, Eli Lily Japan K.K., Boehringer Ingelheim International GmbH, MOCHIDA PHARMACEUTICAL Co., Ltd..
Figures
References
-
- Pavanello C, Calabresi L: Genetic, biochemical, and clinical features of LCAT deficiency: update for 2020. Curr Opin Lipidol, 2020; 31: 232-237 - PubMed
-
- Ishibashi R, Takemoto M, Tsurutani Y, Kuroda M, Ogawa M, Wakabayashi H, Uesugi N, Nagata M, Imai N, Hattori A, Sakamoto K, Kitamoto T, Maezawa Y, Narita I, Hiroi S, Furuta A, Miida T, Yokote K: Immune-mediated acquired lecithin-cholesterol acyltransferase deficiency: A case report and literature review. J Clin Lipidol, 2018; 12: 888-897 e882 - PubMed
-
- Takahashi S, Hiromura K, Tsukida M, Ohishi Y, Hamatani H, Sakurai N, Sakairi T, Ikeuchi H, Kaneko Y, Maeshima A, Kuroiwa T, Yokoo H, Aoki T, Nagata M, Nojima Y: Nephrotic Syndrome Caused by Immune-Mediated Acquired LCAT Deficiency. Journal of the American Society of Nephrology, 2013; 24: 1305-1312 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

