Hepatotoxicity Score: A New Method to Adjust for Use of Potentially Hepatotoxic Medications by Chronic Liver Disease Status
- PMID: 39662972
- PMCID: PMC11634562
- DOI: 10.1002/pds.70069
Hepatotoxicity Score: A New Method to Adjust for Use of Potentially Hepatotoxic Medications by Chronic Liver Disease Status
Abstract
Background: Studies evaluating the hepatic safety of medications have been limited by the inability to control for confounding from receipt of other hepatotoxic drugs.
Objective: The objective of this study was to develop an index (Hepatotoxicity Score) to adjust for concomitant hepatotoxic medication exposure within pharmacoepidemiology studies.
Methods: We identified 193 medications with ≥ 4 reports of hepatotoxicity and created cohorts of outpatient initiators in the Veterans Health Administration (2000-2021). Exposure occurred from initiation through 30 days after discontinuation or up to 1 year. We measured age-/sex-adjusted rates of hospitalization for severe acute liver injury (ALI) by chronic liver disease (CLD), identified drugs with high rates, and used these rates as weights in the score. To demonstrate real-world use, we calculated the score for proton pump inhibitor (PPI) initiators. We summed the weights of the drugs dispensed within 90 days prior to PPI initiation. Hazard ratios (HRs) of severe ALI (95% confidence intervals) were measured with and without adjustment for Hepatotoxicity Score.
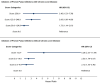
Results: Among 89 512 PPI initiators with CLD, HRs of severe ALI were higher for lansoprazole (HR = 2.17 [95% CI, 1.24-3.82]), but not pantoprazole (HR = 0.83 [95% CI, 0.61-1.13]), versus omeprazole. Adjustment for Hepatotoxicity Score attenuated HRs of lansoprazole (HR = 1.99 [95% CI, 1.13-3.50]). Among 2 462 414 PPI initiators without CLD, HRs were not significantly higher for lansoprazole (HR = 1.66 [95% CI, 0.99-2.77]) but were significantly lower for pantoprazole (HR = 0.59 [95% CI, 0.37-0.95]), versus omeprazole. Adjustment for Hepatotoxicity Score attenuated HRs of lansoprazole (HR = 1.52 [95% CI, 0.91-2.54]).
Conclusions: The Hepatotoxicity Score provides a tool to adjust for confounding due to concomitant hepatotoxic drug exposure within hepatic safety studies.
Keywords: acute liver injury; confounding; hepatotoxicity.
© 2024 The Author(s). Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
S.H. has consulted for Balance Opthalmics Inc.; Lycos Therapeutics Inc.; Applied Therapeutics Inc.; The Medullary Thyroid Cancer Registry Consortium (Novo Nordisk Inc.; AstraZeneca Pharmaceuticals LP, Eli Lilly and Company), Urvant Sciences, i20 Therapeutics, Basilea, Bluebird bio Inc.; Amylyx Pharmaceuticals; Ipsen Bioscience Inc.; Covis Pharma GmbH. V.L.R. has received consulting fees from Entasis, Takeda, and Urovant Sciences.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- P01 AA029545/AA/NIAAA NIH HHS/United States
- U24 AA022001/AA/NIAAA NIH HHS/United States
- U01AA020790/AA/NIAAA NIH HHS/United States
- K08 DK132977/DK/NIDDK NIH HHS/United States
- R01CA206465/CA/NCI NIH HHS/United States
- U24AA022001/AA/NIAAA NIH HHS/United States
- U01 AA020790/AA/NIAAA NIH HHS/United States
- T32 AI055435/AI/NIAID NIH HHS/United States
- U01 AA013566/AA/NIAAA NIH HHS/United States
- T32AI055435/National Institute of Allergy and Infectious Diseases
- K08DK132977/DK/NIDDK NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U01AA013566/AA/NIAAA NIH HHS/United States
- U24AA020794/AA/NIAAA NIH HHS/United States
- R01 CA206465/CA/NCI NIH HHS/United States
- U24 AA020794/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

