Convergence of Type 1 Spiral Ganglion Neuron Subtypes onto Principal Neurons of the Anteroventral Cochlear Nucleus
- PMID: 39663118
- PMCID: PMC11800758
- DOI: 10.1523/JNEUROSCI.1507-24.2024
Convergence of Type 1 Spiral Ganglion Neuron Subtypes onto Principal Neurons of the Anteroventral Cochlear Nucleus
Abstract
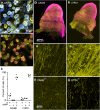
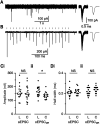
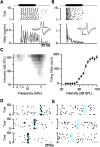
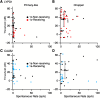
The mammalian auditory system encodes sounds with subtypes of spiral ganglion neurons (SGNs) that differ in sound level sensitivity, permitting discrimination across a wide range of levels. Recent work suggests the physiologically defined SGN subtypes correspond to at least three molecular subtypes. It is not known how information from the different subtypes converges within the cochlear nucleus. We examined this issue using transgenic mice of both sexes that express Cre recombinase in SGNs that are positive for markers of two subtypes: CALB2 (calretinin) in type 1a SGNs and LYPD1 in type 1c SGNs, which correspond to high- and low-sensitivity subtypes, respectively. We crossed these with mice expressing floxed channelrhodopsin, which allowed specific activation of axons from type 1a or 1c SGNs using optogenetics. We made voltage-clamp recordings from bushy cells in the anteroventral cochlear nucleus (AVCN) and found that the synapses formed by CALB2- and LYPD1-positive SGNs had similar EPSC amplitudes and short-term plasticity. Immunohistochemistry revealed that individual bushy cells receive a mix of 1a, 1b, and 1c synapses with VGluT1-positive puncta of similar sizes. We used optogenetic stimulation during in vivo recordings to classify chopper and primary-like units as receiving versus nonreceiving 1a- or 1c-type inputs. These groups showed no significant difference in threshold or spontaneous rate, suggesting the subtypes do not segregate into distinct processing streams in the AVCN. Our results indicate that principal cells in the AVCN integrate information from all SGN subtypes with extensive convergence, which could optimize sound encoding across a large dynamic range.
Keywords: T-stellate cell; bushy cell; cochlear nucleus; endbulb of Held; spiral ganglion.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
