Treatment effect and safety of seltorexant as monotherapy for patients with major depressive disorder: a randomized, placebo-controlled clinical trial
- PMID: 39663378
- PMCID: PMC12092288
- DOI: 10.1038/s41380-024-02846-5
Treatment effect and safety of seltorexant as monotherapy for patients with major depressive disorder: a randomized, placebo-controlled clinical trial
Abstract
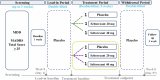
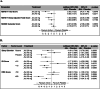
The antidepressant efficacy and safety of seltorexant monotherapy in major depressive disorder (MDD) was investigated in a placebo-controlled, placebo lead-in, randomized, double-blind, phase 1b study. Participants were randomized to receive seltorexant (20 mg or 40 mg) or placebo. The treatment effect was assessed by changes in the Hamilton Rating Scale for Depression-17 item (HDRS17) from treatment-period baseline to week 5 in lead-in placebo non-responders ("enriched" intent-to-treat analysis set). As a secondary outcome, the effect of seltorexant on HDRS17 was assessed in patients with and without subjective insomnia. Seltorexant's effects on polysomnography, serum cortisol, and cortisol waking response were also measured. In total, 128 participants were enrolled, including 86 in the enriched sample (lead-in placebo non-responders). The mean changes from baseline (SD) in HDRS17 score at week 5 differed significantly across arms: -7.0 (5.04) for seltorexant 20 mg, -5.5 (4.34) for seltorexant 40 mg, and -4.4 (3.67) for placebo (p = 0.0456), which was attributable to the difference between the 20 mg and placebo arms (p = 0.0049). Improvement in depression severity at week 5 for seltorexant 20 mg was greater in patients with higher baseline insomnia severity (nominal p = 0.0059). The treatment benefit in the 20 mg arm remained significant when HDRS scores were adjusted by removing the sleep items (nominal p = 0.0289). The mean HDRS17 change versus placebo was numerically larger in the 20 mg than the 40 mg arm, consistent with data from a previous study in which seltorexant was administered adjunctively to conventional antidepressants. In secondary analyses, the waking cortisol response decreased in the 20 mg arm but not the 40 mg or placebo arms, and while total sleep increased more in the 40 mg arm, this arm also showed reduced REM onset latency and increased stage N1 sleep, which were not evident in the 20 mg arm. These biomarker data suggest mechanistic hypotheses that may account for the apparent curvilinear dose-response relationship of seltorexant. Trial Registration: ClinicalTrials.gov, NCT03374475.
© 2024. Janssen Research & Development, LLC, a Johnson & Johnson company.
Conflict of interest statement
Competing interests: This study is supported by Janssen Research and Development. SM, IK, PV, WD, and GP, and are employees of Janssen Research and Development and may hold company stock or stock options. AS and ME are a former employee of Janssen Research and Development and holds stock in the company. HB has received study honoraria from Janssen R&D for conducting the study and is a member of AB for Idorsia, not related to the submitted work.
Figures


References
-
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. - PubMed
-
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

