Neutralizing GDF-15 can overcome anti-PD-1 and anti-PD-L1 resistance in solid tumours
- PMID: 39663448
- PMCID: PMC11779642
- DOI: 10.1038/s41586-024-08305-z
Neutralizing GDF-15 can overcome anti-PD-1 and anti-PD-L1 resistance in solid tumours
Erratum in
-
Author Correction: Neutralizing GDF-15 can overcome anti-PD-1 and anti-PD-L1 resistance in solid tumours.Nature. 2025 Mar;639(8054):E18. doi: 10.1038/s41586-025-08827-0. Nature. 2025. PMID: 40000750 Free PMC article. No abstract available.
Abstract
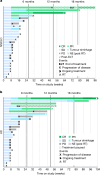
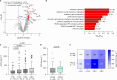
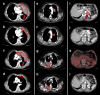
Cancer immunotherapies with antibodies blocking immune checkpoint molecules are clinically active across multiple cancer entities and have markedly improved cancer treatment1. Yet, response rates are still limited, and tumour progression commonly occurs2. Soluble and cell-bound factors in the tumour microenvironment negatively affect cancer immunity. Recently, growth differentiation factor 15 (GDF-15), a cytokine that is abundantly produced by many cancer types, was shown to interfere with antitumour immune response. In preclinical cancer models, GDF-15 blockade synergistically enhanced the efficacy of anti-PD-1-mediated checkpoint inhibition3. In a first-in-human phase 1-2a study (GDFATHER-1/2a trial, NCT04725474 ), patients with advanced cancers refractory to anti-PD-1 or anti-PD-L1 therapy (termed generally as anti-PD-1/PD-L1 refractoriness) were treated with the neutralizing anti-GDF-15 antibody visugromab (CTL-002) in combination with the anti-PD-1 antibody nivolumab. Here we show that durable and deep responses were achieved in some patients with non-squamous non-small cell lung cancer and urothelial cancer, two cancer entities identified as frequently immunosuppressed by GDF-15 in an in silico screening of approximately 10,000 tumour samples in The Cancer Genome Atlas database. Increased levels of tumour infiltration, proliferation, interferon-γ-related signalling and granzyme B expression by cytotoxic T cells were observed in response to treatment. Neutralizing GDF-15 holds promise in overcoming resistance to immune checkpoint inhibition in cancer.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: M.H. and J.W. are co-founders and stock owners of CatalYm. A.d.S., M. Rüdiger, P.L’H., M.H., J.A., A.D., A.A., A.G., F.H., M.A., M.L., D.R., C.S.-W., F.S.L., P.F., K.K., F.H., B.R. and E.L. are current or former employees of and/or hold stock options in Catalym. R.D., M.H., J.W., K.K., E.L., F.H., M. Rüdiger and C.S.-W. are inventors on patents related to visugromab and anti-GDF-15 treatment (WO2014049087, WO2015144855, WO2017055613, WO2022101263, WO2024052532, WO2024126808 and unpublished). I. Melero, H.H., R.B., R.D., J.W., F.J.K., A.M.M.E., W.H.F., T.F.G., P.L., M.M., S.S., A.T. and J.R. have received research funding or consulting or advisory fees from CatalYm. Several authors and investigators declare potentially competing interests (grants or contracts and/or royalties or licences, stock or stock options, consulting fees, honoria, travel or meeting support, or advisory or data safety monitoring board participation) apart from their relationship with CatalYm: I. Melero (Agenus, Alligator, Allmiral, AstraZeneca, Biontech, BMS, Boehringer Ingelheim, Bright Peak, Crescendo Biologics, Curon, Dynamicure, F-Star, Genmab, Highlight Therapeutics, HotSpot, Merck Serono, MSD, Merus, Mestag, Numab, PharmaMar, Pieris, Pierre Fabre, Pioneering Medicines, Roche, Sanofi and Servier); G.d.V. (Astellas, AstraZeneca, Bayer, BMS, Ipsen, Janssen MSD, Merck Serono, Pfizer and Roche); E.G. (Anaveon, BeiGene, Boehringer Ingelheim, Ellipses Pharma, F-Star Therapeutics, Hengrui, Incyte, Janssen Global Services, MabDiscovery, Medscape, MSD, Novartis, Seattle Genetics, Sanofi, Roche, SeaGen, Taiho and Thermo Fisher); J.M.-L. (Astellas, BMS, Highlight Therapeutics, Merck Serono, MSD, Novartis, Ipsen, Roche, Sanofi and Pierre Fabre); M.J. (Adoram, AstraZeneca, Debiopharm, Novartis, Roche, Sanofi and Takeda); M.-E.G. (BMS, Janssen, Novartis and Roche); M.S. (Amgen, AstraZeneca, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Janssen, MSD, Novartis, Roche, Sanofi and Tacalyx); D.K. (AstraZeneca, Amgen, BMS, Merck Serono, MSD, Mirati, PharmaMar, Sanofi and Swiss Oncology in Motion); R.D. (Alligator, Amgen, BMS, MSD, Novartis, Pierre Fabre, Roche, Sun Pharma, Takeda, Sanofi, Second-Genome, Regeneron, T3 Pharma, MaviVax, Pfizer, Simcere and touchIME); M. Reig (AstraZeneca, Bayer, BMS, Boston Scientific, Biotoscana Pharma, Engitix Therapeutics, Eli Lilly, Geneos, Gilead, Ipsen, Merck, Roche and Universal DC); M.-E.R.R. (BMS, Imcore-Roche, Roche and Highlight Therapeutics); E.C. (Achilles, Adcendo, Alkermes, Amunix, Anaveon, Amcure, AstraZeneca, BeiGene, BMS, Boehringer, Chugai, Debiopharm, Diaccurate, Ellipses, Incyte, iTeos, Janssen, Merus, MonTa, MSD, Nanobiotix, Nouscom, Novartis, OncoDNA, PharmaMar, PsiOxus Therapeutics, Roche/Genentech, Sanofi, Servier, Shionogi, Syneos Health, TargImmune, T-knife and Tolremo); J.E.-V. (BMS, MSD and Pfizer); J.J.S.-C. (Eisai, Novartis, Pfizer and Seagen); K.-L.K. (Janssen and Takeda); O.S. (Affimed); C.S. (BMS, Eli Lilly and Boehringer Ingelheim); E.R. (BMS, Eli Lilly, Kyowa Kirin, Leo Pharma, MSD, Pierre Fabre and Sanofi); A.L.-M. (Incyte, Merck, PharmaMar, Pfizer, Roche, Rovi and Sanofi); I. Moreno (Digicore Initiative, Fondacion Intheos, Exafield, Guidepoint and Ellipses Pharma); A.H.-C. (BMS, Gilead, Kyowa Kirin, Merus and MSD); D.H. (Novartis and Roche); M.S.-Z. (Bayer, Instituto de Salud Carlos III (grant FI19/00222), BTG, MSD and Roche); A.V.-L. (AstraZeneca, BMS, Health in Code and Roche); J.T. (Amgen, AstraZeneca, Bristol Myers Squibb, Eisai, EXACT Therapeutics, Institut für Qualitätssicherung und Transparenz im Gesundheitswesen, Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen, Ipsen, Lilly ImClone, medupdate, Merck Serono, Merck Sharp & Dome, Oncolytics Biotech, onkowissen.de, Roche, Servier and streamedup!); C.K. (AstraZenca, BMS, DGVS, Incyte, MCI Deutschland, MSD, Servier, Ipsen, Merck Serono and Roche); P.R.G. (AstraZeneca, Bayer, BMS, Boston Scientific, Eisai, Guerbet, Ipsen, MSD, Lilly, Roche and Sirtex); F.F. (AstraZeneca, BMS, Eisai, Merck Serono, Servier and Roche); Z.T. (Boehringer Ingelheim); H.H. (Secarna); F.J.K. (German Cancer Aid (Deutsche Krebshilfe)); H. Reis (AstraZeneca, BMS, Boehringer Ingelheim, Chop GmbH, Diaceutics, GSK, HUeG, Janssen, MCI, Merck, Novartis, Roche Pharma and Sanofi); M.M. (LYO-X AG and TATAA Biocenter); T.G. (Allogene, Bicara, BMS, Merck, Pyxis and Samyang); W.H.F. (Anaveon, Atreca, Incendia, Ichnos Sciences, Genenta, OSE Immunotherapeutics, Oxford Biotherapeutics, Mestag, Novartis, Roche and Tabby); A.M.M.E. (Acetra, Agenus, BMS, Boehringer Ingelheim, BioInvent, BioNTech, Ellipses, Galecto, GSK, IO Biotech, IQVIA, Isa Pharmaceuticals, Merck & Co, MSD, Pfizer, Pierre Fabre, Sairopa, Sellas, SkylineDX, TigaTx and Trained Immunity); R.B. (Amgen, Avencell); J.R. (Astellas, AstraZeneca, BMS, Daiichi Sankyo, GSK, MSD, Merck and Sanofi); F.H. (Thermosome); P.F. (Curevac); J.W. (Bayrische Forschungsstiftung (FORTiTher) and the German Ministry for Education & Research (Transcan-3)). The remaining authors declare no competing interests.
Figures









References
-
- Lodyga, M. & Hinz, B. TGF-β1 - a truly transforming growth factor in fibrosis and immunity. Semin. Cell Dev. Biol.101, 123–139 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

