Visible Light Triggerable CO Releasing Micelles
- PMID: 39663914
- PMCID: PMC12146008
- DOI: 10.1021/jacs.4c13872
Visible Light Triggerable CO Releasing Micelles
Abstract
Carbon monoxide (CO), along with nitric oxide and hydrogen sulfide, is one of a trinity of known gasotransmitters, or endogenously produced gaseous molecules that signal and regulate a panoply of physiological functions. CO releasing molecules (CORMs) are chemical tools that enable the study and application of this ephemeral gas, that, ideally, release CO on-demand when externally stimulated. Surveying the available triggers, photolysis is potentially advantageous: It is contactless and grants practitioners unparalleled spatial and temporal control. However, current phototriggered CORMs are capricious and do not meet current needs. Presented here is a highly efficient platform for the visible light triggered release of CO gas. This platform is built on a unique CO containing functionality, the cyclopropenone, which undergoes facile decarbonylation through visible light (470 nm) mediated photoredox catalysis. Due to the exothermic strain-release that occurs upon formation of CO, this photoreaction is rapid, quantitative, and has tunable release rates. To render this photo-CORM water-soluble, deliverable, and to keep reactants in proximity, necessary components were polymerized into block copolymers that self-assemble into CO releasing micelles (CORMIs). This platform was compared directly to other state-of-the-art CORMs, showing significantly improved CO production efficiency, lower toxicity, tunable release rates, and consistent efficacy in ex vivo and in vitro settings.
Figures
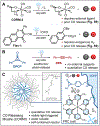


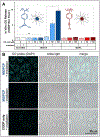
References
-
- Hasegawa U; van der Vlies AJ; Simeoni E; Wandrey C; Hubbell JA Carbon Monoxide-Releasing Micelles for Immunotherapy. J. Am. Chem. Soc 2010, 132 (51), 18273–18280. - PubMed
-
- Zhang X; Yuan Z; Wu J; He Y; Lu G; Zhang D; Zhao Y; Wu R; Lv Y; Cai K; He S. An Orally-Administered Nano-therapeutics with Carbon Monoxide Supplying for Inflammatory Bowel Disease Therapy by Scavenging Oxidative Stress and Restoring Gut Immune Homeostasis. ACS Nano 2023, 17 (21), 21116–21133. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

