Checkpoint based immunotherapy in non-small cell lung cancer: a real-world retrospective study
- PMID: 39664396
- PMCID: PMC11631946
- DOI: 10.3389/fimmu.2024.1419544
Checkpoint based immunotherapy in non-small cell lung cancer: a real-world retrospective study
Abstract
Introduction: Immune checkpoint inhibitor (ICI)-based immunotherapy targeting programmed cell death 1 (PD-1) or its ligand 1 (PD-L1) has radically changed the management of many types of solid tumors including non-small cell lung cancer (NSCLC). Many clinical trials have demonstrated that ICIs improve the survival and the quality of life of patients with advanced non oncogene NSCLC as compared to standard therapies. However, not all patients achieve a clinical benefit from this immunotherapeutic approach. As a result, real-word validation of the efficacy and safety of ICIs can be useful for defining potential predictive biomarkers as well as for overcoming limitations linked to clinical trial restrictions.
Methods: We retrospectively retrieved the clinical data of patients with advanced non oncogene NSCLC treated with ICIs (anti-PD-1 or anti-PD-L1) as single agent or in combination with chemotherapy at "San Giovanni di Dio e Ruggi D'Aragona" University Hospital from January 2016 to December 2023. Potential correlations between clinical-pathological characteristics and safety or survival outcomes were investigated employing the Fisher's exact test, Mann-Whitney U test, the Kruskal-Wallis method and log-rank test, as applicable. Multivariate survival analyses were performed using the Cox proportional hazards model.
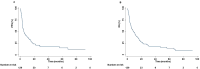
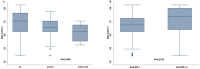
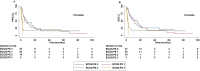
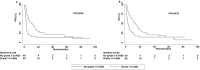
Results: Clinical data of 129 patients were retrieved. At a median follow-up of 29.70 months, progression-free survival (PFS) and overall survival (OS) were 5.27 months and 8.43 months, respectively. At the multivariate analyses, smoking status, presence of bone metastases and the occurrence of immune-related adverse events (irAEs) were correlated with both PFS and OS. Moreover, patients treated with anti-PD-1-based therapy achieved an increased clinical benefit than those treated with anti-PD-L1.
Discussion: In this study we described our real-world experience of ICIs for the treatment of patients with advanced non oncogene NSCLC. A decreased OS in our study population was reported as compared to that of patients included in the clinical trials. Noteworthy, correlations between clinical-pathological characteristics and survival outcomes emerged. Nevertheless, the potential integration of clinical-pathological characteristics as predictive biomarkers in more accurate therapeutic algorithms as well as the underlying biological mechanisms should be further validated in ad hoc studies.
Keywords: ICI; NSCLC; PD-1; PD-L1; biomarker; immunotherapy; predictive; real-world.
Copyright © 2024 Liguori, Giorgio, Polcaro, Pagliara, Malandrino, Perri, Cascella, Ottaiano, Conti, Servetto, Bianco, Pepe and Sabbatino.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures










References
-
- Borghaei H, Gettinger S, Vokes EE, Chow LQM, Burgio MA, de Castro Carpeno J, et al. . Five-year outcomes from the randomized, phase III trials checkMate 017 and 057: nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J Clin Oncol. (2021) 39:723–33. doi: 10.1200/JCO.20.01605 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

