The reporting of disproportionality analysis in pharmacovigilance: spotlight on the READUS-PV guideline
- PMID: 39664518
- PMCID: PMC11632231
- DOI: 10.3389/fphar.2024.1488725
The reporting of disproportionality analysis in pharmacovigilance: spotlight on the READUS-PV guideline
Abstract
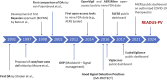
Disproportionality analyses are the most-commonly used study design used in the post-marketing phase to detect suspected adverse drug reactions in individual case safety reports. Recent years have witnessed an exponential increase in published articles on disproportionality analyses, thanks to publicly accessible databases. Unfortunately, this trend was accompanied by concerns on lack of transparency and misinterpretation of results, both generating unjustified alarm and diluting true signals into overwhelming noise. The READUS-PV guideline for reporting disproportionality analysis was developed to tackle this emerging issue. In this perspective article, we describe the rationale behind the development of the READUS-PV guideline, the first collaborative initiative to harmonize the reporting of disproportionality analyses. The adoption of the checklists will assist researchers, regulators, and reviewers in the reporting, assessment, and publication of disproportionality analyses. Acknowledging the challenges ahead of effective implementation, we advocate for a global endorsement by Pharmacology Journals. A wide dissemination of the READUS-PV guideline is crucial to foster transparency and reproducibility of pharmacovigilance research, supporting an effective exploitation of disproportionality analysis among other irreplaceable post-marketing research tools to ensure drug safety.
Keywords: adverse drug reactions; disproportionality analysis; individual case safety reports; pharmacovigilance; signal detection.
Copyright © 2024 Fusaroli, Salvo, Khouri and Raschi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author ER declared that he was an editorial board member of Frontiers in Pharmacology, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Croteau D., Pinnow E., Wu E., Muñoz M., Bulatao I., Dal Pan G. (2022). Sources of evidence triggering and supporting safety-related labeling changes: a 10-year longitudinal assessment of 22 new molecular entities approved in 2008 by the us food and drug administration. Drug Saf. 45, 169–180. 10.1007/s40264-021-01142-3 - DOI - PubMed
-
- Cutroneo P. M., Sartori D., Tuccori M., Crisafulli S., Battini V., Carnovale C., et al. (2024). Conducting and interpreting disproportionality analyses derived from spontaneous reporting systems. Front. Drug Saf. Regul. 3. 10.3389/fdsfr.2023.1323057 - DOI
LinkOut - more resources
Full Text Sources

