Development of Advanced Oral-on-a-Chip: Replicating the Intricate Human Oral Microenvironment
- PMID: 39664582
- PMCID: PMC11628345
- DOI: 10.7150/ijbs.104351
Development of Advanced Oral-on-a-Chip: Replicating the Intricate Human Oral Microenvironment
Abstract
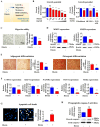
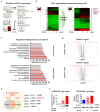
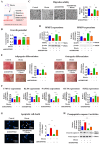
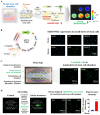
The interactions between various cellular populations in the oral cavity, including gingival keratinocytes, tonsil-resident stem cells, periodontal ligament fibroblasts, and vascular endothelial cells, are crucial for maintaining oral health. These interactions regulate essential functions like tooth support and pathogen defense. However, conventional 2D and 3D in vitro models often fail to capture the complexity of these interactions and the multicellular architecture of the oral environment. To address this limitation, we developed an advanced 3D oral-on-a-chip system that mimics the dynamic microenvironment of oral tissues. This system incorporates multiple oral cells into a 3D structure made from natural polymers such as collagen and hyaluronic acid, crosslinked by blood-coagulating factors. Our study revealed that tonsil-resident stem cells are more sensitive to toxic exposure compared to differentiated cells like fibroblasts and endothelial cells. SERPINB2 was identified as a key biomarker of oral toxicity, with significant upregulation observed in tonsil-resident stem cells after exposure to toxins. Based on this, we developed a fluorescence-linked toxicity detection system using SERPINB2, enabling sensitive and quantitative assessments of oral toxicity. This integrated system provides a valuable tool for evaluating the oral toxicity of drug candidates.
Keywords: Natural polymers; Oral on a chip; SERPINB2; Tonsil-resident stem cells; Toxicity screening.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources

