Repurposing of the Antipsychotic Trifluoperazine Induces SLC7A11/GPX4- Mediated Ferroptosis of Oral Cancer via the ROS/Autophagy Pathway
- PMID: 39664583
- PMCID: PMC11628333
- DOI: 10.7150/ijbs.99859
Repurposing of the Antipsychotic Trifluoperazine Induces SLC7A11/GPX4- Mediated Ferroptosis of Oral Cancer via the ROS/Autophagy Pathway
Abstract
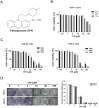
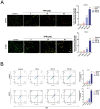
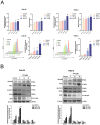
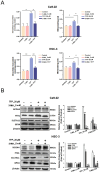
Ferroptosis, a mode of cell death characterized by iron-dependent phospholipid peroxidation, has a substantial therapeutic potential for the treatment of various cancers. This study investigated the effects of trifluoperazine (TFP), an FDA-approved drug traditionally utilized for mental health disorder, on oral cancer cells, with a particular focus on the mechanisms involved in its potential anti-tumor properties. Our findings indicate that TFP significantly elevates the levels of lipid-derived reactive oxygen species (ROS) and induces ferroptotic cell death in oral cancer cells through pathways involving autophagy, the SLC7A11/GPX4 axis, and mitochondrial damage. Additionally, molecular docking analyses revealed that TFP acts as an inhibitor of GPX4. The elevated expression level of GPX4 in oral cancer biopsies was also found to correlate with a poor prognosis. Together, these results provide evidence that TFP selectively induces GPX4-mediated, autophagy-dependent ferroptosis, thereby exerting anti-cancer effects against oral cancer and preventable death.
Keywords: Autophagy; Ferroptosis; Oral Cancer; ROS; Trifluoperazine; mental disorder; preventable death.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures












References
-
- Pekarek L, Garrido-Gil MJ, Sanchez-Cendra A, Cassinello J, Pekarek T, Fraile-Martinez O. et al. Emerging histological and serological biomarkers in oral squamous cell carcinoma: Applications in diagnosis, prognosis evaluation and personalized therapeutics (Review) Oncol Rep. 2023;50:213. - PMC - PubMed
-
- Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. - PubMed
-
- Hsieh MJ, Chen JC, Yang WE, Chien SY, Chen MK, Lo YS. et al. Dehydroandrographolide inhibits oral cancer cell migration and invasion through NF-kappaB-, AP-1-, and SP-1-modulated matrix metalloproteinase-2 inhibition. Biochem Pharmacol. 2017;130:10–20. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

