Integrated electronic health record tools to access real-world data in oncology research
- PMID: 39664645
- PMCID: PMC11633941
- DOI: 10.1093/jamiaopen/ooae144
Integrated electronic health record tools to access real-world data in oncology research
Abstract
Objectives: The Integrating Clinical Trials and Real-World Endpoints (ICAREdata) project aimed to demonstrate that electronic health record (EHR) data, expressed in a standard structured format, can be extracted and transmitted to contribute to clinical research. Using the minimal Common Oncology Data Elements (mCODE), we collected standardized oncology outcome data from EHRs across 10 clinical sites and 15 trials. This report details and assesses the ICAREdata technical implementation and offers recommendations to benefit future projects with similar goals.
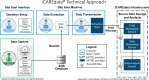
Materials and methods: In the ICAREdata project, we implemented tools to collect structured clinical outcome data within EHRs, then extract and transmit the mCODE-formatted data to a secure cloud storage platform. Using the socio-technical model of health information technology, we systematically assessed the technical implementations in this multi-institutional project.
Results: We evaluated the ICAREdata method across the 8 inter-related dimensions of the socio-technical model. For each dimension, we identified challenges and developed recommendations that implementers of future initiatives can leverage.
Discussion: The ICAREdata project successfully demonstrated the feasibility of using data standards for structured data capture and transmission in clinical trials. The lessons learned from this project can accelerate the success of similar initiatives using standards-based real-world data (RWD) capture and transmission for research.
Conclusion: The ICAREdata project represents a step towards a future where researchers can access high-quality, standardized RWD leading to advances in research and improved care delivery.
Keywords: HL7 FHIR; clinical trials; electronic health records; health information interoperability; real-world data.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Conflict of interest statement
The authors have no competing interests to report.
Figures
Similar articles
-
Feasibility of structuring electronic health record data to facilitate real-world data research: ICAREdata methods applied to multicenter cancer clinical trials.Cancer. 2025 Jan 1;131(1):e35528. doi: 10.1002/cncr.35528. Epub 2024 Aug 28. Cancer. 2025. PMID: 39192753 Free PMC article.
-
Improving Cancer Data Interoperability: The Promise of the Minimal Common Oncology Data Elements (mCODE) Initiative.JCO Clin Cancer Inform. 2020 Oct;4:993-1001. doi: 10.1200/CCI.20.00059. JCO Clin Cancer Inform. 2020. PMID: 33136433 Free PMC article.
-
Data quality and timeliness analysis for post-vaccination adverse event cases reported through healthcare data exchange to FDA BEST pilot platform.Front Public Health. 2024 Jul 8;12:1379973. doi: 10.3389/fpubh.2024.1379973. eCollection 2024. Front Public Health. 2024. PMID: 39040857 Free PMC article.
-
State-of-the-Art Fast Healthcare Interoperability Resources (FHIR)-Based Data Model and Structure Implementations: Systematic Scoping Review.JMIR Med Inform. 2024 Sep 24;12:e58445. doi: 10.2196/58445. JMIR Med Inform. 2024. PMID: 39316433 Free PMC article.
-
HL7 FHIR-based tools and initiatives to support clinical research: a scoping review.J Am Med Inform Assoc. 2022 Aug 16;29(9):1642-1653. doi: 10.1093/jamia/ocac105. J Am Med Inform Assoc. 2022. PMID: 35818340 Free PMC article.
Cited by
-
Challenges in Automating Extraction of Real-World Radiographic Images and Adverse Events: Lessons From the ICAREdata Initiative.JCO Clin Cancer Inform. 2025 Apr;9:e2400319. doi: 10.1200/CCI-24-00319. Epub 2025 Apr 18. JCO Clin Cancer Inform. 2025. PMID: 40249879 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources