Evaluation of alternative vaccination routes against paratuberculosis in goats
- PMID: 39664904
- PMCID: PMC11631874
- DOI: 10.3389/fvets.2024.1457849
Evaluation of alternative vaccination routes against paratuberculosis in goats
Abstract
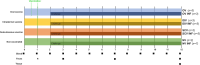
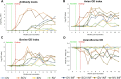
Paratuberculosis is a chronic granulomatous enteritis, caused by Mycobacterium avium subspecies paratuberculosis (Map), that affects ruminants worldwide. Vaccination has been considered the most cost-effective method for the control of this disease in infected dairy herds. However, currently available vaccines do not provide complete protection and interfere with the diagnosis of both paratuberculosis and bovine tuberculosis, limiting its use. Because of that, efforts are being made for the development of new vaccines. The primary objective of this study was to evaluate the efficacy of two whole-cell inactivated experimental vaccines against paratuberculosis in goats, administered through the oral (OV) and intradermal (IDV) routes, and compare them with that of the commercial subcutaneous vaccine Gudair® (SCV). Over an 11-month period, the effect of vaccination and a subsequent Map challenge on the specific peripheral immune responses and Map-DNA fecal shedding were recorded. At the end of the experiment, tissue bacterial load and lesion severity were assessed. The experimental vaccines did not induce specific humoral immune responses and only elicited mild and delayed cellular immune responses. Although the OV reduced lesion severity, neither this vaccine nor the IDV prototype was able to reduce fecal shedding or tissue bacterial load. Moreover, although the SCV did not confer sterile immunity, it outperformed both experimental vaccines in all these parameters.
Keywords: goat; immunization strategies; intradermal vaccine; oral vaccine; paratuberculosis.
Copyright © 2024 Criado, Silva, Arteche-Villasol, Zapico, Elguezabal, Molina, Espinosa, Ferreras, Benavides, Pérez and Gutiérrez-Expósito.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Economic analysis of Mycobacterium avium subspecies paratuberculosis vaccines in dairy herds.J Dairy Sci. 2012 Apr;95(4):1855-72. doi: 10.3168/jds.2011-4787. J Dairy Sci. 2012. PMID: 22459833
-
Evaluation of novel oral vaccine candidates and validation of a caprine model of Johne's disease.Front Cell Infect Microbiol. 2014 Mar 4;4:26. doi: 10.3389/fcimb.2014.00026. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24624365 Free PMC article. Clinical Trial.
-
Effect of heat-inactivated Mycobacterium avium subspecies paratuberculosis (MAP) vaccine on the lesions and immunopathology developed in target tissues of naturally MAP-infected goats.Vet Microbiol. 2022 Oct;273:109543. doi: 10.1016/j.vetmic.2022.109543. Epub 2022 Aug 8. Vet Microbiol. 2022. PMID: 36037619
-
Vaccination against paratuberculosis.Expert Rev Vaccines. 2008 Aug;7(6):817-32. doi: 10.1586/14760584.7.6.817. Expert Rev Vaccines. 2008. PMID: 18665779 Review.
-
A rational framework for evaluating the next generation of vaccines against Mycobacterium avium subspecies paratuberculosis.Front Cell Infect Microbiol. 2014 Sep 9;4:126. doi: 10.3389/fcimb.2014.00126. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25250245 Free PMC article. Review.
References
-
- WOAH . Chapter 3.1.16. Paratuberculosis (Johne’s disease) In: Manual of diagnostic tests and vaccines for terrestrial animals. Paris, France: World Organisation for Animal Health (WOAH). (2024).
-
- More S, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, et al. . Assessment of listing and categorisation of animal diseases within the framework of the animal health law (regulation (EU) no 2016/429): paratuberculosis. EFSA J. (2017) 15:e04960. doi: 10.2903/j.efsa.2017.4960, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

