SUrvey of renal Biopsy registry database and Anticancer dRUg therapy in Japan (SUBARU-J study)
- PMID: 39664993
- PMCID: PMC11630032
- DOI: 10.1093/ckj/sfae327
SUrvey of renal Biopsy registry database and Anticancer dRUg therapy in Japan (SUBARU-J study)
Abstract
Background: Kidney complications associated with anticancer drug therapy have greatly increased recently. We aimed to investigate the real-world clinical outcomes of anticancer drug therapy-associated renal complications in Japan using the national kidney biopsy database, Japan Renal Biopsy Registry (J-RBR).
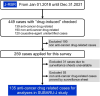
Methods: From 2018 to 2021, 449 cases from 49 facilities identified as 'drug-induced' histopathology in the J-RBR were screened, of which a total of 135 were confirmed as anticancer drug-related cases and included in the analysis. Overall survival rates were estimated using the Kaplan-Meier method and compared by logrank test. The Cox regression model was used to evaluate the association between variables and deaths.
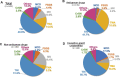
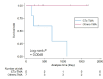
Results: The most common primary sites of malignancies were the lung (33.3%), followed by gastrointestinal (16.3%) and gynaecological (11.1%) cancers. Tubulointerstitial nephritis (TIN; 47.4%) and thrombotic microangiopathy (TMA; 35.6%) were the most frequent diagnoses. All immunoglobulin A nephropathy, minimal change disease and crescentic glomerulonephritis (CrGN) cases were immune checkpoint inhibitor related. All CrGN cases were anti-neutrophil cytoplasmic antibody negative. Antibiotics were most frequently used concomitantly with anticancer drugs in TMA cases among subgroups (TMA versus others: 62.5 versus 27.5%; P < .001). Among TMA cases, the serum lactate dehydrogenase level tended to be higher in cytotoxic agent-associated TMA (CTx-TMA) than in other TMAs, but was not significant between groups (415.5 versus 219.0 U/l; P = .06). Overall survival was worse in CTx-TMA than in other TMAs (P = .007). The Cox model demonstrated proton pump inhibitor (PPI) use (hazard ratio 2.49, P = .001) as a significant prognostic factor, as well as the presence of metastasis and serum albumin level.
Conclusions: Our registry analysis highlighted various presentations of biopsy-proven kidney complications associated with anticancer drug therapy. Clinicians should be aware of worse outcomes associated with CTx-TMA and the prognostic role of PPI use.
Keywords: PPI use; anticancer drug therapy; kidney biopsy registry database; thrombotic microangiopathy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
None declared.
Figures


References
LinkOut - more resources
Full Text Sources

