High-risk cytogenetic abnormalities in multiple myeloma: PETHEMA-GEM experience
- PMID: 39665068
- PMCID: PMC11632121
- DOI: 10.1002/hem3.70031
High-risk cytogenetic abnormalities in multiple myeloma: PETHEMA-GEM experience
Abstract
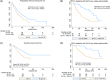
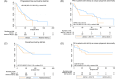
This study examines the impact of cytogenetic abnormalities and their co-segregation on the prognosis of newly diagnosed multiple myeloma patients. The analysis included 1304 patients from four different GEM-PETHEMA clinical trials. Genetic alterations, such as t(4;14), t(14;16), del(17p), +1q, and del(1p), were investigated using FISH on CD38 purified plasma cells. The frequency of genetic alterations detected were as follows: del(17p) in 8%, t(4;14) in 12%, t(14;16) in 3%, +1q in 43%, and del(1p) in 8%. The median follow-up was 61 months, and the median progression-free survival (PFS) and overall survival (OS) were 44 months and not reached, respectively. Consistent with previous reports, the presence of t(4;14) was associated with shorter PFS and OS. In our series, the presence of t(14;16) did not impact survival, maybe due to limitations in sample size. Del(17p) was linked to poor prognosis using a cut-off level of ≥20% positive cells, without any impact of higher cut-off in prognosis, except for patients with clonal fraction ≥80% who had a dismal outcome. Cosegregation of cytogenetic abnormalities patients worsened the prognosis in t(4;14) patients but not in patients with del(17p), which retained its adverse prognosis even as a solitary abnormality. Gain(1q) was associated with significantly shorter PFS and OS, while del(1p) affected PFS but not OS. Nevertheless, when co-segregation was eliminated, the detrimental effect of +1q or del(1p) was no longer observed. In conclusion, this study confirms the prognostic significance of high-risk cytogenetic abnormalities in MM and highlights the importance of considering co-occurrence for accurate prognosis assessment.
© 2024 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
Veronica González‐Calle: Consulting or Advisory Role: Janssen. Speakers' Bureau: Janssen, GlaxoSmithKline, Pfizer, Bristol Myers Squibb/Celgene. Travel, Accommodations, Expenses: Janssen, GlaxoSmithKline. Paula Rodriguez‐Otero declares honoraria derived from Consulting or advisory board role: Celgene‐BMS, Janssen, Roche, Abbvie, Pfizer, GSK, Sanofi, H3Biomedicine. Steering committee member: Celgene‐BMS, Regeneron, Janssen Speaker's bureau: Janssen, Celgene‐BMS, GSK, Sanofi, Abbvie. Travel grant: Pfizer, and is an Editor of HemaSphere. Miguel T. Hernández: Consulting or Advisory Role: Janssen, Sanofi/Aventis, Celgene/Bristol Myers Squibb, GlaxoSmithKline. Speakers' Bureau: Janssen, Celgene/Bristol Myers Squibb, GlaxoSmithKline. Research Funding: Celgene/Bristol Myers Squibb (Inst). Laura Rosiñol reports honoraria from Janssen, Celgene, Amgen, and Takeda. Joaquin Martínez‐López declares honoraria from consulting activities from Astellas Pharma, BMS, F. Hoffman‐La Roche, Janssen, Novartis, Sanofi. Research grant from BMS. Ana P. González‐Rodríguez reports honoraria from Janssen, Celgene, Takeda, and Amgen. JdlR is a consultant for Bristol‐Meyers Squibb, GlaxoSmithKline, Janssen, Menarini, Pfizer, Sanofi, and Takeda and is on the advisory board of GlaxoSmithKline, Janssen, Pfizer, and Sanofi. Maria V. Mateos: declares honoraria derived from lectures and advisory roles at Amgen, Celgene, BMS, GSK, Janssen, Pfizer, Regeneron, Sanofi, and Takeda. Anna Sureda reports consultancy for BMS, Celgene, Gilead, Janssen, MSD, Novartis, Sanofi, Takeda, and GSK; speakers bureau for Takeda; travel grants from BMS, Celgene, Janssen, Roche, Sanofi, and Takeda; research support from Takeda and BMS. Albert Oriol declares consultant activities from Amgen, Celgene, BMS, GSK, Janssen, and Sanofi. EMO reports consulting or an advisory role for Amgen, AbbVie, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Karyopharm, Menarini‐Stemline, Mundipharma, Oncopeptides, Sanofi, Secura Bio, and Takeda; meeting and/or travel expenses from Bristol Myers Squibb, GlaxoSmithKline, Janssen, Lilly, and Sanofi; and honoraria from Amgen, Asofarma, Bristol Myers Squibb, GlaxoSmithKline, Janssen, MSD, Pfizer, Sanofi, and Takeda. Joan Bargay: honoraria for lectures and advisory boards from Janssen, BMS‐Celgene, Amgen, Takeda, Oncopeptides. Juan J. Lahuerta: Consulting or Advisory Role: Celgene, Amgen, Janssen‐Cilag, Sanofi. Travel, Accommodations, Expenses: Celgene, Pfizer. Jesus F. San Miguel declares consulting activities from Abbvie, Amgen, BMS, Celgene, F. Hoffman‐La Roche, GSK, HaemaLogiX, KaryoPharm, Merck, Novartis, Pfizer, Regeneron, Sanofi‐Aventis, Takeda, and SecuraBio. Maria V. Mateos: honoraria for lectures and advisory boards from Janssen, Celgene, Takeda, Amgen, GSK, Abbvie, Pfizer, Regeneron, Roche, Sanofi, Oncopeptides; The remaining authors declare no competing interests relative to this work.
Figures



References
-
- Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12(5):335‐348. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
