Atlas of Cerebrospinal Fluid Immune Cells Across Neurological Diseases
- PMID: 39665159
- PMCID: PMC11889529
- DOI: 10.1002/ana.27157
Atlas of Cerebrospinal Fluid Immune Cells Across Neurological Diseases
Abstract
Objective: Cerebrospinal fluid (CSF) provides unique insights into the brain and neurological diseases. However, the potential of CSF flow cytometry applied on a large scale remains unknown.
Methods: We used data-driven approaches to analyze paired CSF and blood flow cytometry measurements from 8,790 patients (discovery cohort) and CSF only data from 3,201 patients (validation cohort) collected across neurological diseases in a real-world setting.
Results: In somatoform controls (n = 788), activation of T cells increased with age in both CSF and blood, whereas double negative blood T cells (CD3+CD4-CD8-) decreased with age. A machine learning model of CSF and blood immune cells defined immune age, which correlated strongly with true biological age (r = 0.71). Classifying all diseases solely based on the CSF/blood parameters in 8,790 patients resulted in clusters of 4 disease categories: healthy, autoimmune, meningoencephalitis, and neurodegenerative. This clustering was validated in an analytically independent test dataset (8,790 patients) and in a temporally independent cohort (3,201 patients). Patients with multiple sclerosis were more likely to have progressive disease when assigned to the neurodegeneration cluster and to have lower disability in the autoimmune cluster. Patients with dementia in the neurodegeneration cluster showed more severe disease progression. Flow cytometry helped differentiate dementia from controls, thereby enhancing the diagnostic power of routine CSF diagnostics.
Interpretation: Flow cytometry of CSF and blood thus identifies site-specific aging patterns and disease-overarching patterns of neurodegeneration. ANN NEUROL 2025;97:779-790.
© 2024 The Author(s). Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
All authors declare no competing interest.
Figures


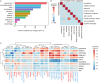


References
-
- Deisenhammer F, Bartos A, Egg R, et al. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur J Neurol 2006;13:913–922. - PubMed
-
- Kowarik MC, Grummel V, Wemlinger S, et al. Immune cell subtyping in the cerebrospinal fluid of patients with neurological diseases. J Neurol 2014;261:130–143. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

