Histopathologic Features and Transcriptomic Signatures Do Not Solve the Issue of Magnetic Resonance Imaging-Invisible Prostate Cancers: A Matched-Pair Analysis
- PMID: 39665170
- PMCID: PMC11776441
- DOI: 10.1002/pros.24838
Histopathologic Features and Transcriptomic Signatures Do Not Solve the Issue of Magnetic Resonance Imaging-Invisible Prostate Cancers: A Matched-Pair Analysis
Abstract
Background: Multiparametric magnetic resonance imaging (mpMRI) is pivotal in prostate cancer (PCa) diagnosis, but some clinically significant (cs) PCa remain undetected. This study aims to understand the pathological and molecular basis for csPCa visibility at mpMRI.
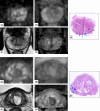
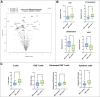
Methods: We performed a retrospective matched-pair cohort study, including patients undergoing radical prostatectomy (RP) for csPCa (i.e., ISUP grade group ≥ 2) from 2015 to 2020, in our tertiary-referral center. We screened for inclusion in the "mpMRI-invisible" cohort all consecutive men (N = 45) having a negative preoperative mpMRI. The "mpMRI-visible" cohort was matched based on age, PSA, prostate volume, ISUP grade group. Included patients underwent radiological and pathological open-label revisions and characterization of the tumor mRNA expression profile (analyzing 780 gene transcripts, signaling pathways, and cell-type profiling). We compared the clinical-pathological variables and the gene expression profile between matched pairs. The analysis was stratified according to histological characteristics and lesion diameter.
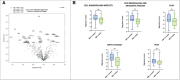
Results: We included 34 patients (17 per cohort); mean age at RP and PSA were 70.5 years (standard deviation [SD] = 7.7), 7.1 ng/mL (SD = 3.3), respectively; 65% of men were ISUP 2. Overall, no significant differences in histopathological features, tumor diameter and location, mRNA profile, pathways, and cell-type scores emerged between cohorts. In the stratified analysis, an upregulation of cell adhesion and motility, of extracellular matrix remodeling and of metastatic process pathways was present in specific subgroups of mpMRI-invisible cancers.
Conclusions: No PCa pathological or gene-expression hallmarks explaining mp-MRI invisibility were identified. Aggressive features can be present both in mpMRI-invisible and -visible tumors.
Keywords: MRI invisible; diagnostic imaging; expression profiling; genomic; nanostring; prostate cancer (clinically significant).
© 2024 The Author(s). The Prostate published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Mottet N., Cornford P., R. C. N. den van Bergh, , et al., EAU Guidelines (Edn. Presented at the EAU Annual Congress Milan 2023) (Arnhem, The Netherlands: EAU Guidelines Office, 2023), http://uroweb.org/guidelines/compilations-of-all-guidelines/.
-
- Ahmed H. U., El‐Shater Bosaily A., Brown L. C., et al., “Diagnostic Accuracy of Multi‐Parametric MRI and TRUS Biopsy in Prostate Cancer (PROMIS): A Paired Validating Confirmatory Study,” Lancet 389 (2017): 815–822. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

