TEAD1-Mediated Trans-Differentiation of Vascular Smooth Muscle Cells into Fibroblast-Like Cells Contributes to the Stabilization and Repair of Disrupted Atherosclerotic Plaques
- PMID: 39665254
- PMCID: PMC11791998
- DOI: 10.1002/advs.202407408
TEAD1-Mediated Trans-Differentiation of Vascular Smooth Muscle Cells into Fibroblast-Like Cells Contributes to the Stabilization and Repair of Disrupted Atherosclerotic Plaques
Abstract
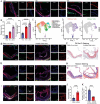
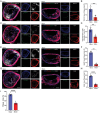
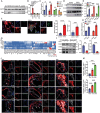
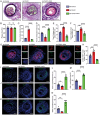
Atherosclerotic plaque rupture mainly contributes to acute coronary syndrome (ACS). Insufficient repair of these plaques leads to thrombosis and subsequent ACS. Central to this process is the modulation of vascular smooth muscle cells (VSMCs) phenotypes, emphasizing their pivotal role in atherosclerotic plaque stability and healing post-disruption. Here, an expansion of FSP1+ cells in a tandem stenosis (TS) model of atherosclerotic mice is unveiled, predominantly originating from VSMCs through single-cell RNA sequencing (scRNA-seq) analyses and VSMC lineage tracing studies. Further investigation identified TEA domain transcription factor 1 (TEAD1) as the key transcription factor driving the trans-differentiation of VSMCs into fibroblast-like cells. In vivo experiments using a TS model of plaque rupture demonstrated that TEAD1 played a crucial role in promoting plaque stability and healing post-rupture through pharmacological or TEAD1-AAV treatments. Mechanistically, it is found that TEAD1 promoted the expression of fibroblast markers through the Wnt4/β-Catenin pathway, facilitating the trans-differentiation. Thus, this study illustrated that TEAD1 played a critical role in promoting the trans-differentiation of VSMCs into fibroblast-like cells and subsequent extracellular matrix production through the Wnt4/β-Catenin pathway. Consequently, this process enhanced the healing mechanisms following plaque rupture, elucidating potential therapeutic avenues for managing atherosclerotic instability.
Keywords: ACS; TEAD1; atherosclerotic plaque; plaque rupture; thrombosis.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ahmadi A., Argulian E., Leipsic J., Newby D. E., Narula J., J. Am. Coll. Cardiol. 2019, 74, 1608. - PubMed
-
- Gaba P., Gersh B. J., Muller J., Narula J., Stone G. W., Nat. Rev. Cardiol. 2023, 20, 181. - PubMed
-
- Montone R. A., Camilli M., Russo M., Termite C., La Vecchia G., Iannaccone G., Rinaldi R., Gurgoglione F., Del Buono M. G., Sanna T., Trani C., Liuzzo G., Crea F., Niccoli G., JACC Cardiovasc. Imaging 2022, 15, 325. - PubMed
-
- Rashid I., Maghzal G. J., Chen Y.‐C., Cheng D., Talib J., Newington D., Ren M., Vajandar S. K., Searle A., Maluenda A., Lindstedt E.‐L., Jabbour A., Kettle A. J., Bongers A., Power C., Michaëlsson E., Peter K., Stocker R., Eur. Heart J. 2018, 39, 3301. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
